Cloning, expression and functional activity of deoxyhypusine synthase from Plasmodium vivax
- PMID: 17042947
- PMCID: PMC1654163
- DOI: 10.1186/1471-2180-6-91
Cloning, expression and functional activity of deoxyhypusine synthase from Plasmodium vivax
Abstract
Background: Plasmodium vivax is the most widespread human malaria parasite. However, genetic information about its pathogenesis is limited at present, due to the lack of a reproducible in vitro cultivation method. Sequencing of the Plasmodium vivax genome suggested the presence of a homolog of deoxyhypusine synthase (DHS) from P. falciparum, the key regulatory enzyme in the first committed step of hypusine biosynthesis. DHS is involved in cell proliferation, and thus a valuable drug target for the human malaria parasite P. falciparum. A comparison of the enzymatic properties of the DHS enzymes between the benign and severe Plasmodium species should contribute to our understanding of the differences in pathogenicity and phylogeny of both malaria parasites.
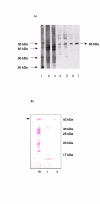
Results: We describe the cloning of a 1368 bp putative deoxyhypusine synthase gene (dhs) sequence from genomic DNA of P. vivax PEST strain Salvador I (Accession number AJ549098) after touchdown PCR. The corresponding protein was expressed and functionally characterized as deoxyhypusine synthase by determination of its specific activity and cross-reactivity to human DHS on a Western blot. The putative DHS protein from P. vivax displays a FASTA score of 75 relative to DHS from rodent malaria parasite, P. yoelii, and 74 relative to that from the human parasite, P. falciparum strain 3D7. The ORF encoding 456 amino acids was expressed under control of IPTG-inducible T7 promoter, and expressed as a protein of approximately 50 kDa (theoretically 52.7 kDa) in E. coli BL21 DE3 cells. The N-terminal histidine-tagged protein was purified by Nickel-chelate affinity chromatography under denaturing conditions. DHS with a theoretical pI of 6.0 was present in both eluate fractions. The specific enzymatic activity of DHS was determined as 1268 U/mg protein. The inhibitor, N-guanyl-1, 7-diaminoheptane (GC7), suppressed specific activity by 36-fold. Western blot analysis performed with a polyclonal anti-human DHS antibody revealed cross-reactivity to DHS from P. vivax, despite an amino acid identity of 44% between the proteins.
Conclusion: We identify a novel DHS protein in the more benign malaria parasite,P. vivax, on the basis of specific enzymatic activity, cross-reactivity with a polyclonal antibody against human DHS, and amino acid identity with DHS homologs from the rodent malaria parasite, P. yoelii, and human P. falciparum strains.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

