Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice
- PMID: 17043104
- PMCID: PMC1828418
- DOI: 10.1128/IAI.01217-06
Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice
Abstract
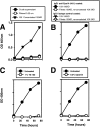
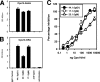
Exosomes activate T cells in vivo, but whether exosomes are able to induce humoral immune responses is still unknown. We found that dendritic cells, but not other immune cells, constitutively release an exosome-associated glycoconjugate that is cross-reactive with the capsular polysaccharide of Streptococcus pneumoniae type 14 (Cps14-CRA). Cps14-CRA was localized to the cholesterol-enriched microdomains or rafts of the exosomes and was mapped to the beta1-->6 branched N-acetyl-lactosamine derivatives of the Cps14-CRA. Injection of CFA-primed naive mice with purified dendritic cell exosomes induced immunoglobulin (Ig) anti-Cps14 responses composed predominantly of IgM, IgG3, and IgG1. These responses were associated with protection against a lethal challenge with live S. pneumoniae type 14, but not with type 3 bacteria, and was correlated with the titer of elicited IgM and IgG3 anti-Cps14. These data show, for the first time, that exosomes can induce a humoral immune response to an associated unprocessed, autologous antigen. Although anti-Cps14 Ig responses are specifically demonstrated, these could reflect a broader mechanism that modulates both natural immunity and autoimmunity to other glycotopes.
Figures


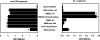
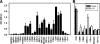


Similar articles
-
Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1.J Immunol. 2002 Apr 1;168(7):3437-43. doi: 10.4049/jimmunol.168.7.3437. J Immunol. 2002. PMID: 11907102
-
Peptide mimics of two pneumococcal capsular polysaccharide serotypes (6B and 9V) protect mice from a lethal challenge with Streptococcus pneumoniae.Eur J Immunol. 2009 Jun;39(6):1527-35. doi: 10.1002/eji.200839091. Eur J Immunol. 2009. PMID: 19499518
-
Distinct approaches to investigate the importance of the murine 4-1BB 4-1BBL interaction in the antibody response to Streptococcus pneumoniae.J Leukoc Biol. 2007 Sep;82(3):638-44. doi: 10.1189/jlb.1006628. Epub 2007 Jun 5. J Leukoc Biol. 2007. PMID: 17550973
-
Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae.Trends Immunol. 2001 Jun;22(6):308-11. doi: 10.1016/s1471-4906(01)01926-3. Trends Immunol. 2001. PMID: 11377289 Review.
-
Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae.Curr Protein Pept Sci. 2006 Aug;7(4):295-305. doi: 10.2174/138920306778017972. Curr Protein Pept Sci. 2006. PMID: 16918444 Review.
Cited by
-
Using exosomes, naturally-equipped nanocarriers, for drug delivery.J Control Release. 2015 Dec 10;219:396-405. doi: 10.1016/j.jconrel.2015.07.030. Epub 2015 Aug 1. J Control Release. 2015. PMID: 26241750 Free PMC article. Review.
-
Exosomal miRNAs in the plasma of Cynoglossus semilaevis infected with Vibrio harveyi: Pleiotropic regulators and potential biomarkers involved in inflammatory and immune responses.Front Immunol. 2022 Aug 18;13:949670. doi: 10.3389/fimmu.2022.949670. eCollection 2022. Front Immunol. 2022. PMID: 36059498 Free PMC article.
-
An immunoregulatory role of dendritic cell-derived exosomes versus HIV-1 infection: take it easy but be warned.Ann Transl Med. 2017 Sep;5(17):362. doi: 10.21037/atm.2017.06.34. Ann Transl Med. 2017. PMID: 28936456 Free PMC article. No abstract available.
-
Transfer of extracellular vesicles during immune cell-cell interactions.Immunol Rev. 2013 Jan;251(1):125-42. doi: 10.1111/imr.12013. Immunol Rev. 2013. PMID: 23278745 Free PMC article. Review.
-
Evaluation of the inflammatory response in macrophages stimulated with exosomes secreted by Mycobacterium avium-infected macrophages.Biomed Res Int. 2015;2015:658421. doi: 10.1155/2015/658421. Epub 2015 Mar 16. Biomed Res Int. 2015. PMID: 25861639 Free PMC article.
References
-
- Acosta-Rodriguez, E. V., C. L. Montes, C. C. Motran, E. I. Zuniga, F. T. Liu, G. A. Rabinovich, and A. Gruppi. 2004. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 172:493-502. - PubMed
-
- Alonso De Velasco, E., B. A. Dekker, A. F. Verheul, R. G. Feldman, J. Verhoef, and H. Snippe. 1995. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J. Infect. Dis. 172:562-565. - PubMed
-
- Andre, F., N. Chaput, N. E. Schartz, C. Flament, N. Aubert, J. Bernard, F. Lemonnier, G. Raposo, B. Escudier, D. H. Hsu, T. Tursz, S. Amigorena, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172:2126-2136. - PubMed
-
- Briles, D. E., C. Forman, S. Hudak, and J. L. Claflin. 1984. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J. Mol. Cell. Immunol. 1:305-309. - PubMed
-
- Chaput, N., N. E. Schartz, F. Andre, J. Taieb, S. Novault, P. Bonnaventure, N. Aubert, J. Bernard, F. Lemonnier, M. Merad, G. Adema, M. Adams, M. Ferrantini, A. F. Carpentier, B. Escudier, T. Tursz, E. Angevin, and L. Zitvogel. 2004. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J. Immunol. 172:2137-2146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

