Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers
- PMID: 17043123
- PMCID: PMC1694003
- DOI: 10.1128/AAC.00494-06
Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers
Abstract
The CS-023 concentration in plasma after administration by infusion to healthy volunteers at a dose of 700 mg was decreased, with a half-life of 1.7 h, and the cumulative urinary excretion was 59.4% of the dose. The total clearance, renal clearance, and volume of distribution were 8.12 liters/h, 4.14 liters/h, and 17.2 liters, respectively.
Figures
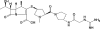

Similar articles
-
Effect of impaired renal function on the pharmacokinetics of tomopenem (RO4908463/CS-023), a novel carbapenem.Antimicrob Agents Chemother. 2008 Jul;52(7):2360-6. doi: 10.1128/AAC.01249-07. Epub 2008 Apr 28. Antimicrob Agents Chemother. 2008. PMID: 18443123 Free PMC article. Clinical Trial.
-
Prediction of pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, in humans using animal data.Xenobiotica. 2007 Jan;37(1):91-102. doi: 10.1080/00498250601047889. Xenobiotica. 2007. PMID: 17178636
-
Pharmacokinetics and disposition of CS-023 (RO4908463), a novel parenteral carbapenem, in animals.Antimicrob Agents Chemother. 2007 Jan;51(1):257-63. doi: 10.1128/AAC.00459-06. Epub 2006 Oct 30. Antimicrob Agents Chemother. 2007. PMID: 17074792 Free PMC article.
-
Lack of age and gender effects on single-dose pharmacokinetics of tomopenem (RO4908463/CS-023), a novel carbapenem.Br J Clin Pharmacol. 2009 Apr;67(4):469-72. doi: 10.1111/j.1365-2125.2009.03367.x. Br J Clin Pharmacol. 2009. PMID: 19371322 Free PMC article. Clinical Trial.
-
[Pharmacokinetics of carbapenems].Klin Mikrobiol Infekc Lek. 2012 Jun;18(3):68-74. Klin Mikrobiol Infekc Lek. 2012. PMID: 22786828 Review. Czech.
Cited by
-
Effect of impaired renal function on the pharmacokinetics of tomopenem (RO4908463/CS-023), a novel carbapenem.Antimicrob Agents Chemother. 2008 Jul;52(7):2360-6. doi: 10.1128/AAC.01249-07. Epub 2008 Apr 28. Antimicrob Agents Chemother. 2008. PMID: 18443123 Free PMC article. Clinical Trial.
-
Efficacy of human-simulated exposures of tomopenem (formerly CS-023) in a murine model of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus infection.Antimicrob Agents Chemother. 2011 Nov;55(11):5004-9. doi: 10.1128/AAC.00068-11. Epub 2011 Aug 15. Antimicrob Agents Chemother. 2011. PMID: 21844314 Free PMC article.
-
Potent in vitro activity of tomopenem (CS-023) against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2008 Aug;52(8):2849-54. doi: 10.1128/AAC.00413-08. Epub 2008 Jun 2. Antimicrob Agents Chemother. 2008. PMID: 18519723 Free PMC article.
-
Pharmacodynamics of the antibacterial effect and emergence of resistance to tomopenem, formerly RO4908463/CS-023, in an in vitro pharmacokinetic model of Staphylococcus aureus infection.Antimicrob Agents Chemother. 2008 Apr;52(4):1401-6. doi: 10.1128/AAC.01153-07. Epub 2008 Jan 28. Antimicrob Agents Chemother. 2008. PMID: 18227179 Free PMC article.
-
In vitro activity of tomopenem (CS-023/RO4908463) against anaerobic bacteria.Antimicrob Agents Chemother. 2009 Jan;53(1):319-22. doi: 10.1128/AAC.00595-08. Epub 2008 Oct 27. Antimicrob Agents Chemother. 2009. PMID: 18955522 Free PMC article.
References
-
- Bax, R. P., W. Bastain, A. Featherstone, D. M. Wilkinson, M. Hutchison, and S. J. Haworth. 1989. The pharmacokinetics of meropenem in volunteers. J. Antimicrob. Chemother. 24(Suppl. A):311-320. - PubMed
-
- Craig, W. A. 1997. The pharmacology of meropenem, a new carbapenem antibiotic. Clin. Infect. Dis. 24(Suppl. 2):S266-S275. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. - PubMed
-
- Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. - PubMed
-
- Drusano, G. L., and H. C. Standiford. 1985. Pharmacokinetic profile of imipenem/cilastatin in normal volunteers. Am. J. Med. 78(Suppl. 6A):47-53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

