Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly
- PMID: 17043135
- PMCID: PMC2064574
- DOI: 10.1083/jcb.200602125
Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly
Abstract
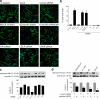
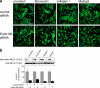
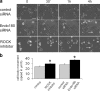
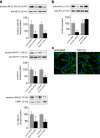
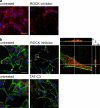
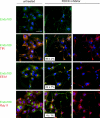
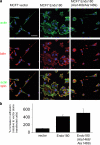
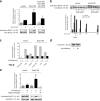
The regulated assembly and disassembly of focal adhesions and adherens junctions contributes to cell motility and tumor invasion. Pivotal in this process is phosphorylation of myosin light chain-2 (MLC2) by Rho kinase (ROCK) downstream of Rho activation, which generates the contractile force necessary to drive disassembly of epithelial cell-cell junctions and cell-matrix adhesions at the rear of migrating cells. How Rho-ROCK-MLC2 activation occurs at these distinct cellular locations is not known, but the emerging concept that endocytic dynamics can coordinate key intracellular signaling events provides vital clues. We report that endosomes containing the promigratory receptor Endo180 (CD280) can generate Rho-ROCK-MLC2-based contractile signals. Moreover, we provide evidence for a cellular mechanism in which Endo180-containing endosomes are spatially localized to facilitate their contractile signals directly at sites of adhesion turnover. We propose migration driven by Endo180 as a model for the spatial regulation of contractility and adhesion dynamics by endosomes.
Figures

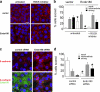
References
-
- Behrendt, N. 2004. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biol. Chem. 385:103–136. - PubMed
-
- Behrendt, N., O.N. Jensen, L.H. Engelholm, E. Mortz, M. Mann, and K. Dano. 2000. A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 275:1993–2002. - PubMed
-
- Carragher, N.O., and M.C. Frame. 2004. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14:241–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

