Dynamic subcompartmentalization of the mitochondrial inner membrane
- PMID: 17043137
- PMCID: PMC2064565
- DOI: 10.1083/jcb.200605138
Dynamic subcompartmentalization of the mitochondrial inner membrane
Abstract
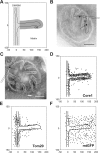
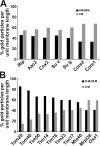
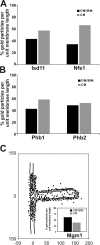
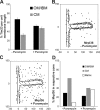
The inner membrane of mitochondria is organized in two morphologically distinct domains, the inner boundary membrane (IBM) and the cristae membrane (CM), which are connected by narrow, tubular cristae junctions. The protein composition of these domains, their dynamics, and their biogenesis and maintenance are poorly understood at the molecular level. We have used quantitative immunoelectron microscopy to determine the distribution of a collection of representative proteins in yeast mitochondria belonging to seven major processes: oxidative phosphorylation, protein translocation, metabolite exchange, mitochondrial morphology, protein translation, iron-sulfur biogenesis, and protein degradation. We show that proteins are distributed in an uneven, yet not exclusive, manner between IBM and CM. The individual distributions reflect the physiological functions of proteins. Moreover, proteins can redistribute between the domains upon changes of the physiological state of the cell. Impairing assembly of complex III affects the distribution of partially assembled subunits. We propose a model for the generation of this dynamic subcompartmentalization of the mitochondrial inner membrane.
Figures


References
-
- Ardail, D., J.P. Privat, M. Egret-Charlier, C. Levrat, F. Lerme, and P. Louisot. 1990. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265:18797–18802. - PubMed
-
- Bornhovd, C., F. Vogel, W. Neupert, and A.S. Reichert. 2006. Mitochondrial membrane potential is dependent on the oligomeric state of F1FO-ATP synthase supracomplexes. J. Biol. Chem. 281:13990–13998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

