SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER
- PMID: 17043138
- PMCID: PMC2064567
- DOI: 10.1083/jcb.200605196
SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER
Abstract
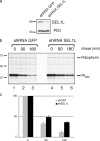
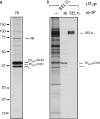
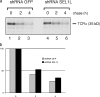
Protein quality control in the endoplasmic reticulum (ER) involves recognition of misfolded proteins and dislocation from the ER lumen into the cytosol, followed by proteasomal degradation. Viruses have co-opted this pathway to destroy proteins that are crucial for host defense. Examination of dislocation of class I major histocompatibility complex (MHC) heavy chains (HCs) catalyzed by the human cytomegalovirus (HCMV) immunoevasin US11 uncovered a conserved complex of the mammalian dislocation machinery. We analyze the contributions of a novel complex member, SEL1L, mammalian homologue of yHrd3p, to the dislocation process. Perturbation of SEL1L function discriminates between the dislocation pathways used by US11 and US2, which is a second HCMV protein that catalyzes dislocation of class I MHC HCs. Furthermore, reduction of the level of SEL1L by small hairpin RNA (shRNA) inhibits the degradation of a misfolded ribophorin fragment (RI332) independently of the presence of viral accessories. These results allow us to place SEL1L in the broader context of glycoprotein degradation, and imply the existence of multiple independent modes of extraction of misfolded substrates from the mammalian ER.
Figures

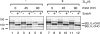


References
-
- Ahner, A., and J.L. Brodsky. 2004. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 14:474–478. - PubMed
-
- Bays, N.W., R.G. Gardner, L.P. Seelig, C.A. Joazeiro, and R.Y. Hampton. 2001. a. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3:24–29. - PubMed
-
- Bebok, Z., C. Mazzochi, S.A. King, J.S. Hong, and E.J. Sorscher. 1998. The mechanism underlying cystic fibrosis transmembrane conductance regulator transport from the endoplasmic reticulum to the proteasome includes Sec61beta and a cytosolic, deglycosylated intermediary. J. Biol. Chem. 273:29873–29878. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

