Membranes of the world unite!
- PMID: 17043140
- PMCID: PMC2064561
- DOI: 10.1083/jcb.200607083
Membranes of the world unite!
Abstract
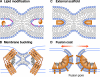
Despite diverse origins, cellular fusion mechanisms converge at a pathway of phospholipid bilayer fusion. In this mini-review, we discuss how proteins can mediate each of the three major stages in the fusion pathway: contact, hemifusion, and the opening of an expanding fusion pore.
Figures

References
-
- Barona, T., R.D. Byrne, T.R. Pettitt, M.J. Wakelam, B. Larijani, and D. Poccia. 2005. Diacylclycerol induces fusion of nuclear envelope membrane precursor vesicles. J. Biol. Chem. 280:41171–41177. - PubMed
-
- Chernomordik, L.V., and M.M. Kozlov. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72:175–207. - PubMed
-
- Chernomordik, L.V., and M.M. Kozlov. 2005. Membrane hemifusion: crossing a chasm in two leaps. Cell. 123:375–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

