Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction
- PMID: 17043150
- PMCID: PMC2151582
- DOI: 10.1085/jgp.200609638
Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction
Abstract
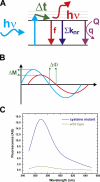
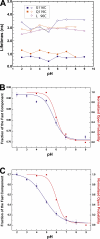
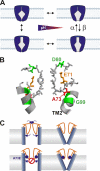
The closed KcsA channel structure revealed a crossing of the cytosolic ends of the transmembrane helices blocking the permeation pathway. It is generally agreed that during channel opening this helical bundle crossing has to widen in order to enable access to the inner cavity. Here, we address the question of whether the opening of the inner gate is sufficient for ion conduction, or if a second gate, located elsewhere, may interrupt the ion flow. We used fluorescence lifetime measurements on KcsA channels labeled with tetramethylrhodamine at residues in the C-terminal end of TM2 to report on the opening of the lower pore region. We found two populations of channels with different fluorescence lifetimes, whose relative distribution agrees with the open probability of the channel. The absolute fraction of channels found with an open bundle crossing is too high to explain the low open probability of the KcsA-WT channel. We found the same distribution as in the WT channel between open and closed bundle crossing for two KcsA mutants, A73E and E71A, which significantly increase open probability at low pH. These two results strongly suggest that a second gate in the ion permeation pathway exists. The location of the mutations A73E and E71A suggests that the second gate may be the selectivity filter, which resides in an inactivated state under steady-state conditions. Since the long closed times observed in KcsA-WT are not present in KcsA-A73E or -E71A, we propose that KcsA-WT remains predominantly in a state with an open bundle crossing but closed (inactivated) second gate, while the mutations A73E and E71A sharply decrease the tendency to enter in the inactivated state, and as a consequence, the second gate is predominantly open at steady state. The ability to monitor the opening of the bundle crossing optically enables the direct recording of the movement of the pore helices while the channel is functioning.
Figures




References
-
- Cha, A., and F. Bezanilla. 1997. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron. 19:1127–1140. - PubMed

