Positive charges at the intracellular mouth of the pore regulate anion conduction in the CFTR chloride channel
- PMID: 17043152
- PMCID: PMC2151590
- DOI: 10.1085/jgp.200609516
Positive charges at the intracellular mouth of the pore regulate anion conduction in the CFTR chloride channel
Abstract
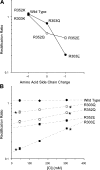
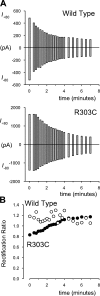
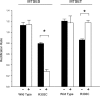
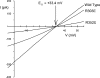
Many different ion channel pores are thought to have charged amino acid residues clustered around their entrances. The so-called surface charges contributed by these residues can play important roles in attracting oppositely charged ions from the bulk solution on one side of the membrane, increasing effective local counterion concentration and favoring rapid ion movement through the channel. Here we use site-directed mutagenesis to identify arginine residues contributing important surface charges in the intracellular mouth of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channel pore. While wild-type CFTR was associated with a linear current-voltage relationship with symmetrical solutions, strong outward rectification was observed after mutagenesis of two arginine residues (R303 and R352) located near the intracellular ends of the fifth and sixth transmembrane regions. Current rectification was dependent on the charge present at these positions, consistent with an electrostatic effect. Furthermore, mutagenesis-induced rectification was more pronounced at lower Cl(-) concentrations, suggesting that these mutants had a reduced ability to concentrate Cl(-) ions near the inner pore mouth. R303 and R352 mutants exhibited reduced single channel conductance, especially at negative membrane potentials, that was dependent on the charge of the amino acid residue present at these positions. However, the very low conductance of both R303E and R352E-CFTR could be greatly increased by elevating intracellular Cl(-) concentration. Modification of an introduced cysteine residue at position 303 by charged methanethiosulfonate reagents reproduced charge-dependent effects on current rectification. Mutagenesis of arginine residues in the second and tenth transmembrane regions also altered channel permeation properties, however these effects were not consistent with changes in channel surface charges. These results suggest that positively charged arginine residues act to concentrate Cl(-) ions at the inner mouth of the CFTR pore, and that this contributes to maximization of the rate of Cl(-) ion permeation through the pore.
Figures

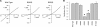
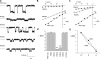

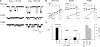
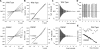

References
-
- Anderson, M.P., R.J. Gregory, S. Thompson, D.W. Souza, S. Paul, R.C. Mulligan, A.E. Smith, and M.J. Welsh. 1991. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 253:202–205. - PubMed

