Powering the planet: chemical challenges in solar energy utilization
- PMID: 17043226
- PMCID: PMC1635072
- DOI: 10.1073/pnas.0603395103
Powering the planet: chemical challenges in solar energy utilization
Erratum in
- Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):20142
Abstract
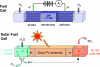
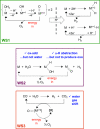
Global energy consumption is projected to increase, even in the face of substantial declines in energy intensity, at least 2-fold by midcentury relative to the present because of population and economic growth. This demand could be met, in principle, from fossil energy resources, particularly coal. However, the cumulative nature of CO(2) emissions in the atmosphere demands that holding atmospheric CO(2) levels to even twice their preanthropogenic values by midcentury will require invention, development, and deployment of schemes for carbon-neutral energy production on a scale commensurate with, or larger than, the entire present-day energy supply from all sources combined. Among renewable energy resources, solar energy is by far the largest exploitable resource, providing more energy in 1 hour to the earth than all of the energy consumed by humans in an entire year. In view of the intermittency of insolation, if solar energy is to be a major primary energy source, it must be stored and dispatched on demand to the end user. An especially attractive approach is to store solar-converted energy in the form of chemical bonds, i.e., in a photosynthetic process at a year-round average efficiency significantly higher than current plants or algae, to reduce land-area requirements. Scientific challenges involved with this process include schemes to capture and convert solar energy and then store the energy in the form of chemical bonds, producing oxygen from water and a reduced fuel such as hydrogen, methane, methanol, or other hydrocarbon species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
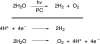
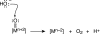

References
-
- Energy Information Administration. Annual Energy Outlook. Washington, DC: US Dept of Energy; 2005.
-
- Nakicenovic N, Swart R, editors. Special Report on Emissions Scenarios. Washington, DC: Intergovernmental Panel on Climate Change; 2000. pp. 48–55.
-
- Kates R. Environment. 2000;42:10–19.
-
- Hoffert MI, Caldeira K, Jain AK, Haites EF, Harvey LD, Potter SD, Schlesinger ME, Wigley TML, Wuebbles DJ. Nature. 1998;395:881–884.
-
- United Nations Development Program. World Energy Assessment Report: Energy and the Challenge of Sustainability. New York: United Nations; 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

