Global aggregation of newly translated proteins in an Escherichia coli strain deficient of the chaperonin GroEL
- PMID: 17043235
- PMCID: PMC1613232
- DOI: 10.1073/pnas.0607534103
Global aggregation of newly translated proteins in an Escherichia coli strain deficient of the chaperonin GroEL
Abstract
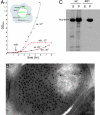
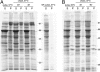
In a newly isolated temperature-sensitive lethal Escherichia coli mutant affecting the chaperonin GroEL, we observed wholesale aggregation of newly translated proteins. After temperature shift, transcription, translation, and growth slowed over two to three generations, accompanied by filamentation and accretion (in approximately 2% of cells) of paracrystalline arrays containing mutant chaperonin complex. A biochemically isolated inclusion body fraction contained the collective of abundant proteins of the bacterial cytoplasm as determined by SDS/PAGE and proteolysis/MS analyses. Pulse-chase experiments revealed that newly made proteins, but not preexistent ones, were recruited to this insoluble fraction. Although aggregation of "stringent" GroEL/GroES-dependent substrates may secondarily produce an "avalanche" of aggregation, the observations raise the possibility, supported by in vitro refolding experiments, that the widespread aggregation reflects that GroEL function supports the proper folding of a majority of newly translated polypeptides, not just the limited number indicated by interaction studies and in vitro experiments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Georgopoulos CP, Hendrix RW, Kaiser AD. Nat New Biol. 1972;239:38–41. - PubMed
-
- Takano T, Kakefuda T. Nat New Biol. 1972;239:34–37. - PubMed
-
- Cheng M-Y, Hartl FU, Martin J, Pollock RA, Kalousek F, Neupert W, Hallberg EM, Hallberg RL, Horwich AL. Nature. 1989;337:620–625. - PubMed
-
- Ostermann J, Horwich AL, Neupert W, Hartl FU. Nature. 1989;341:125–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

