Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis
- PMID: 17043238
- PMCID: PMC1613228
- DOI: 10.1073/pnas.0607423103
Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis
Abstract
The most common inherited form of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease affecting adult motoneurons, is caused by dominant mutations in the ubiquitously expressed Cu(2+)/Zn(2+) superoxide dismutase (SOD1). Recent studies suggest that glia may contribute to motoneuron injury in animal models of familial ALS. To determine whether the expression of mutant SOD1 (mSOD1(G93A)) in CNS microglia contributes to motoneuron injury, PU.1(-/-) mice that are unable to develop myeloid and lymphoid cells received bone marrow transplants resulting in donor-derived microglia. Donor-derived microglia from mice overexpressing mSOD1(G93A), an animal model of familial ALS, transplanted into PU.1(-/-) mice could not induce weakness, motoneuron injury, or an ALS-like disease. To determine whether expression of mSOD1(G93A) in motoneurons and astroglia, as well as microglia, was required to produce motoneuron disease, PU.1(-/-) mice were bred with mSOD1(G93A) mice. In mSOD1(G93A)/PU.1(-/-) mice, wild-type donor-derived microglia slowed motoneuron loss and prolonged disease duration and survival when compared with mice receiving mSOD1(G93A) expressing cells or mSOD1(G93A) mice. In vitro studies confirmed that wild-type microglia were less neurotoxic than similarly cultured mSOD1(G93A) microglia. Compared with wild-type microglia, mSOD1(G93A) microglia produced and released more superoxide and nitrite+nitrate, and induced more neuronal death. These data demonstrate that the expression of mSOD1(G93A) results in activated and neurotoxic microglia, and suggests that the lack of mSOD1(G93A) expression in microglia may contribute to motoneuron protection. This study confirms the importance of microglia as a double-edged sword, and focuses on the importance of targeting microglia to minimize cytotoxicity and maximize neuroprotection in neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
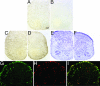
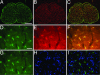

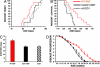

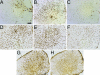
References
-
- Cleveland DW, Rothstein JD. Nat Rev Neurosci. 2001;2:806–819. - PubMed
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Nature. 1993;362:59–62. - PubMed
-
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Science. 1994;264:1772–1775. - PubMed
-
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. Neuron. 1995;14:1105–1116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

