Management of overactive bladder with transdermal oxybutynin
- PMID: 17043706
- PMCID: PMC1578546
Management of overactive bladder with transdermal oxybutynin
Abstract
In clinical trials, transdermal oxybutynin (OXY-TDS) has shown comparable efficacy and improved tolerability when compared with conventional pharmacotherapy. Systemic anticholinergic adverse effects are comparable to those with placebo, most likely owing to avoidance of first-pass hepatic metabolism and conversion of oxybutynin to N-desethyloxybutynin. OXY-TDS has predictable pharmacokinetic absorption and elimination parameters, as shown in both in vitro and in vivo studies. Consistent plasma concentrations of oxybutynin avoid labile peak and trough concentrations seen with immediate-release formulations, paralleling extended-release drug delivery. This novel drug delivery system has unique dermatologic skin application site reactions, including erythema and pruritus. Skin reactions are usually mild and can be minimized by varying the site of patch application. Most eczematous dermatologic reactions can be appropriately treated with a moderately potent topical corticosteroid cream. A small percentage of patients will discontinue therapy as a result of bothersome application site skin reactions.
Figures

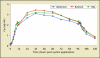
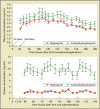
Similar articles
-
Treatments for overactive bladder: focus on pharmacotherapy.J Obstet Gynaecol Can. 2012 Nov;34(11):1092-1101. doi: 10.1016/S1701-2163(16)35440-8. J Obstet Gynaecol Can. 2012. PMID: 23231848
-
Pharmacokinetics and metabolism of transdermal oxybutynin: in vitro and in vivo performance of a novel delivery system.Pharm Res. 2003 Jan;20(1):103-9. doi: 10.1023/a:1022259011052. Pharm Res. 2003. PMID: 12608543 Clinical Trial.
-
Development of oxybutynin chloride topical gel for overactive bladder.Open Access J Urol. 2011 Apr 4;3:35-42. doi: 10.2147/OAJU.S17046. Open Access J Urol. 2011. PMID: 24198634 Free PMC article. Review.
-
An update on the use of transdermal oxybutynin in the management of overactive bladder disorder.Ther Adv Urol. 2016 Apr;8(2):83-90. doi: 10.1177/1756287215626312. Epub 2016 Jan 19. Ther Adv Urol. 2016. PMID: 27034721 Free PMC article. Review.
-
Transdermal oxybutynin in the treatment of adults with overactive bladder: combined results of two randomized clinical trials.World J Urol. 2005 Sep;23(4):263-70. doi: 10.1007/s00345-005-0012-8. Epub 2005 Nov 8. World J Urol. 2005. PMID: 16151816 Clinical Trial.
Cited by
-
Oxybutynin topical gel in the treatment of overactive bladder.Open Access J Urol. 2010 Jun 16;2:91-8. Open Access J Urol. 2010. PMID: 24198618 Free PMC article. Review.
-
First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring.Drug Deliv. 2023 Dec;30(1):2180113. doi: 10.1080/10717544.2023.2180113. Drug Deliv. 2023. PMID: 36815245 Free PMC article.
-
A comparative review of oxybutynin chloride formulations: pharmacokinetics and therapeutic efficacy in overactive bladder.Rev Urol. 2010 Winter;12(1):12-9. Rev Urol. 2010. PMID: 20428289 Free PMC article.
-
Transdermal oxybutynin.Drugs. 2009;69(3):327-37. doi: 10.2165/00003495-200969030-00008. Drugs. 2009. PMID: 19275276 Review.
-
An Updated Structure of Oxybutynin Hydrochloride.bioRxiv [Preprint]. 2024 Jun 6:2024.06.05.597682. doi: 10.1101/2024.06.05.597682. bioRxiv. 2024. Update in: Adv Sci (Weinh). 2024 Oct;11(40):e2406494. doi: 10.1002/advs.202406494. PMID: 38895300 Free PMC article. Updated. Preprint.
References
-
- Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60(5) suppl 1:7–12. - PubMed
-
- Burgio KL, Ives DG, Locher JL, et al. Treatment seeking for urinary incontinence in older adults. J Am Geriatr Soc. 1994;42:208–212. - PubMed
-
- Dugan E, Roberts CP, Cohen SJ, et al. Why older community-dwelling adults do not discuss urinary incontinence with their primary care physicians. J Am Geriatr Soc. 2001;49:462–465. - PubMed
-
- Ouslander JG. Management of overactive bladder. N Engl J Med. 2004;350:786–799. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
