Distinctive gene expression of prostatic stromal cells cultured from diseased versus normal tissues
- PMID: 17044071
- PMCID: PMC2732006
- DOI: 10.1002/jcp.20828
Distinctive gene expression of prostatic stromal cells cultured from diseased versus normal tissues
Abstract
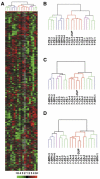
To obtain a comprehensive view of the transcriptional programs in prostatic stromal cells of different histological/pathological origin, we profiled 18 adult human stromal cell cultures from normal transition zone (TZ), normal peripheral zone (PZ), benign prostatic hyperplasia (BPH), and prostate cancer (CA) using cDNA microarrays. A hierarchical clustering analysis of 714 named unique genes whose expression varied at least threefold from the overall mean abundance in at least three samples in all 18 samples demonstrated that cells of different origin displayed distinct gene expression profiles. Many of the differentially expressed genes are involved in biological processes known to be important in the development of prostatic diseases including cell proliferation and apoptosis, cell adhesion, and immune response. Significance Analysis of Microarrays (SAM) analysis identified genes that showed differential expression with statistical significance including 24 genes between cells from TZ versus BPH, 34 between BPH versus CA, and 101 between PZ versus CA. S100A4 and SULF1, the most up- and downregulated genes in BPH versus TZ, respectively, showed expression at the protein level consistent with microarray analysis. In addition, sulfatase assay showed that BPH cells have lower SULF1 activity compared to TZ cells. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis confirmed differential expression of ENPP2/autotoxin and six other genes between PZ versus CA, as well as differential expression of six genes between BPH versus CA. Our results support the hypothesis that prostatic stromal cells of different origin have unique transcriptional programs and point towards genes involved in actions of stromal cells in BPH and CA.
Figures




References
-
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. - PubMed
-
- Black EJ, Clair T, Delrow J, Neiman P, Gillespie DA. Microarray analysis identifies Autotaxin, a tumour cell motility and angiogenic factor with lysophospholipase D activity, as a specific target of cell transformation by v-Jun. Oncogene. 2004;23(13):2357–2366. - PubMed
-
- Brindley DN. Lipid phosphate phosphatases and related proteins: Signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92(5):900–912. - PubMed
-
- Brooks JD. Microarray analysis in prostate cancer research. Curr Opin Urol. 2002;12(5):395–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

