Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis
- PMID: 17044733
- PMCID: PMC1564427
- DOI: 10.1371/journal.ppat.0020092
Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis
Abstract
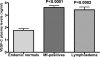
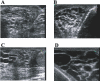
Lymphatic filariasis is a disease of considerable socioeconomic burden in the tropics. Presently used antifilarial drugs are able to strongly reduce transmission and will thus ultimately lower the burden of morbidity associated with the infection, however, a chemotherapeutic principle that directly induces a halt or improvement in the progression of the morbidity in already infected individuals would constitute a major lead. In search of such a more-effective drug to complement the existing ones, in an area endemic for bancroftian filariasis in Ghana, 33 microfilaremic and 18 lymphedema patients took part in a double-blind, placebo-controlled trial of a 6-wk regimen of 200 mg/day doxycycline. Four months after doxycycline treatment, all patients received 150-200 microg/kg ivermectin and 400 mg albendazole. Patients were monitored for Wolbachia and microfilaria loads, antigenemia, filarial dance sign (FDS), dilation of supratesticular lymphatic vessels, and plasma levels of lymphangiogenic factors (vascular endothelial growth factor-C [VEGF-C] and soluble vascular endothelial growth factor receptor-3 [(s)VEGFR-3]). Lymphedema patients were additionally monitored for stage (grade) of lymphedema and the circumferences of affected legs. Wolbachia load, microfilaremia, antigenemia, and frequency of FDS were significantly reduced in microfilaremic patients up to 24 mo in the doxycycline group compared to the placebo group. The mean dilation of supratesticular lymphatic vessels in doxycycline-treated patients was reduced significantly at 24 mo, whereas there was no improvement in the placebo group. Preceding clinical improvement, at 12 mo, the mean plasma levels of VEGF-C and sVEGFR-3 decreased significantly in the doxycycline-treated patients to a level close to that of endemic normal values, whereas there was no significant reduction in the placebo patients. The extent of disease in lymphedema patients significantly improved following doxycycline, with the mean stage of lymphedema in the doxycycline-treated patients being significantly lower compared to placebo patients 12 mo after treatment. The reduction in the stages manifested as better skin texture, a reduction of deep folds, and fewer deep skin folds. In conclusion, a 6-wk regimen of antifilarial treatment with doxycycline against W. bancrofti showed a strong macrofilaricidal activity and reduction in plasma levels of VEGF-C/sVEGFR-3, the latter being associated with amelioration of supratesticular dilated lymphatic vessels and with an improvement of pathology in lymphatic filariasis patients.
Conflict of interest statement
Figures




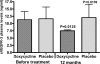



References
-
- World Health Organization. WHO annual report on lymphatic filariasis 2004. 2005. Available: http://www.filariasis.org. Accessed 12 October 2005.
-
- Cox FE. Elimination of lymphatic filariasis as a public health problem. Parasitol Today. 2000;16:135. - PubMed
-
- Noroes J, Dreyer G, Santos A, Mendes VG, Medeiros Z, et al. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans R Soc Trop Med Hyg. 1997;91:78–81. - PubMed
-
- Bockarie MJ, Tisch DJ, Kastens W, Alexander ND, Dimber Z, et al. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–1848. - PubMed
-
- Meyrowitsch DW, Simonsen PE, Magesa SM. Long-term effect of three different strategies for mass diethylcarbamazine administration in bancroftian filariasis: Follow-up at 10 years after treatment. Trans R Soc Trop Med Hyg. 2004;98:627–634. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

