Different prion disease phenotypes result from inoculation of cattle with two temporally separated sources of sheep scrapie from Great Britain
- PMID: 17044917
- PMCID: PMC1636635
- DOI: 10.1186/1746-6148-2-31
Different prion disease phenotypes result from inoculation of cattle with two temporally separated sources of sheep scrapie from Great Britain
Abstract
Background: Given the theoretical proposal that bovine spongiform encephalopathy (BSE) could have originated from sheep scrapie, this study investigated the pathogenicity for cattle, by intracerebral (i.c.) inoculation, of two pools of scrapie agents sourced in Great Britain before and during the BSE epidemic. Two groups of ten cattle were each inoculated with pools of brain material from sheep scrapie cases collected prior to 1975 and after 1990. Control groups comprised five cattle inoculated with sheep brain free from scrapie, five cattle inoculated with saline, and for comparison with BSE, naturally infected cattle and cattle i.c. inoculated with BSE brainstem homogenate from a parallel study. Phenotypic characterisation of the disease forms transmitted to cattle was conducted by morphological, immunohistochemical, biochemical and biological methods.
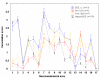
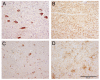
Results: Disease occurred in 16 cattle, nine inoculated with the pre-1975 inoculum and seven inoculated with the post-1990 inoculum, with four cattle still alive at 83 months post challenge (as at June 2006). The different inocula produced predominantly two different disease phenotypes as determined by histopathological, immunohistochemical and Western immunoblotting methods and biological characterisation on transmission to mice, neither of which was identical to BSE. Whilst the disease presentation was uniform in all scrapie-affected cattle of the pre-1975 group, the post-1990 inoculum produced a more variable disease, with two animals sharing immunohistochemical and molecular profile characteristics with animals in the pre-1975 group.
Conclusion: The study has demonstrated that cattle inoculated with different pooled scrapie sources can develop different prion disease phenotypes, which were not consistent with the phenotype of BSE of cattle and whose isolates did not have the strain typing characteristics of the BSE agent on transmission to mice.
Figures









References
-
- Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987;121:419–420. - PubMed
-
- Wilesmith JW, Wells GA. Bovine spongiform encephalopathy. Curr Top Microbiol Immunol. 1991;172:21–38. - PubMed
-
- Simmons MM, Harris P, Jeffrey M, Meek SC, Blamire IWH, Wells GAH. BSE in Great Britain: Consistency of the neurohistopathological findings in two random annual samples of clinically suspect cases. Vet Rec. 1996;138:175–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

