Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility
- PMID: 17046690
- PMCID: PMC6116893
- DOI: 10.1016/j.neuron.2006.07.023
Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility
Abstract
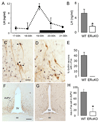
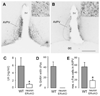
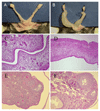
The mechanisms through which estrogen regulates gonadotropin-releasing hormone (GnRH) neurons to control mammalian ovulation are unknown. We found that estrogen positive feedback to generate the preovulatory gonadotropin surge was normal in estrogen receptor beta knockout (ERbeta) mutant mice, but absent in ERalpha mutant mice. An ERalpha-selective compound was sufficient to generate positive feedback in wild-type mice. As GnRH neurons do not express ERalpha, estrogen positive feedback upon GnRH neurons must be indirect in nature. To establish the cell type responsible, we generated a neuron-specific ERalpha mutant mouse line. These mice failed to exhibit estrogen positive feedback, demonstrating that neurons expressing ERalpha are critical. We then used a GnRH neuron-specific Pseudorabies virus (PRV) tracing approach to show that the ERalpha-expressing neurons innervating GnRH neurons are located within rostral periventricular regions of the hypothalamus. These studies demonstrate that ovulation is driven by estrogen actions upon ERalpha-expressing neuronal afferents to GnRH neurons.
Figures
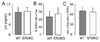

References
-
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. - PubMed
-
- Card JP, Enquist LW. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. - PubMed
-
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

