Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward
- PMID: 17046698
- PMCID: PMC2826987
- DOI: 10.1016/j.neuron.2006.08.009
Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward
Abstract
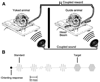
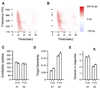
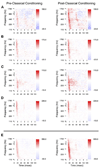
We tested the involvement of cognition in adult experience-dependent neuroplasticity using primate cortical implants. In a prior study, learning an operant sensory discrimination increased cortical excitability and target selectivity. Here, the prior task was separated into three behavioral phases. First, naive animals were exposed to stimulus-reward pairings from the prior study. These yoked animals did not have to discriminate to be rewarded and did not learn the discrimination. The plasticity observed in the prior study did not occur. Second, the animals were classically conditioned to discriminate the same stimuli in a simplified format. Learning was accompanied by increased sensory response strength and an increased range of sensory inputs eliciting responses. The third study recreated the original operant discrimination, and selectivity for task targets increased. These studies demonstrate that cognitive association between sensory stimuli and reinforcers accompanies adult experience-dependent cortical plasticity and suggest that selectivity in representation and action are linked.
Figures





References
-
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J. Neurophysiol. 1991;66:1048–1058. - PubMed
-
- Bakin J, South D, Weinberger N. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav. Neurosci. 1996;110:905–913. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

