Endonuclease G promotes cell death of non-invasive human breast cancer cells
- PMID: 17046751
- PMCID: PMC1839947
- DOI: 10.1016/j.yexcr.2006.09.012
Endonuclease G promotes cell death of non-invasive human breast cancer cells
Abstract
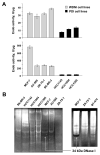
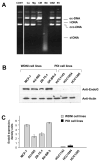
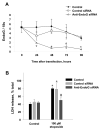
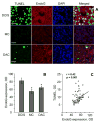
The invasiveness of breast cancer cells was shown to be associated with the suppressed ability to develop apoptosis. The role of cell death DNases/endonucleases has not been previously examined in relation with the invasiveness of breast cancer cells. We have compared the activity of the endonucleases in seven human breast cancer cell lines different in the level of invasiveness and differentiation. The invasiveness of cell lines was confirmed by an in vitro Matrigel-based assay. The total endonuclease activity in the differentiated non-invasive (WDNI) cell lines was higher than that in the poorly differentiated invasive (PDI) cells. The expression of EndoG strongly correlated with the degree of estrogen receptor expression and showed an inverse correlation with vimentin and matrix metalloproteinase-13. The EndoG-positive WDNI cells were more sensitive to etoposide- or camptothecin-induced cell death than EndoG-negative PDI cells. Silencing of EndoG caused inhibited of SK-BR-3 WDNI cell death induced by etoposide. Human ductal carcinomas in situ expressed high levels of EndoG, while invasive medullar and ductal carcinomas had significantly decreased expression of EndoG. This correlated with decreased apoptosis as measured by TUNEL assay. Our findings suggest that the presence of EndoG in non-invasive breast cancer cells determines their sensitivity to apoptosis, which may be taken into consideration for developing the chemotherapeutic strategy for cancer treatment.
Figures



References
-
- Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–44. - PubMed
-
- Meyer JS, Koehm SL, Hughes JM, Higa E, Wittliff JL, Lagos JA, Manes JL. Bromodeoxyuridine labeling for S-phase measurement in breast carcinoma. Cancer. 1993;71:3531–40. - PubMed
-
- Kim MS, Kang HJ, Moon A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch Pharm Res. 2001;24:349–54. - PubMed
-
- Fernandez Y, Gu B, Martinez A, Torregrosa A, Sierra A. Inhibition of apoptosis in human breast cancer cells: role in tumor progression to the metastatic state. Int J Cancer. 2002;101:317–26. - PubMed
-
- Mommers EC, van Diest PJ, Leonhart AM, Meijer CJ, Baak JP. Balance of cell proliferation and apoptosis in breast carcinogenesis. Breast Cancer Res Treat. 1999;58:163–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

