Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats
- PMID: 17047164
- PMCID: PMC3034291
- DOI: 10.1152/ajprenal.00175.2006
Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats
Abstract
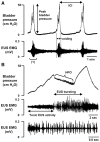
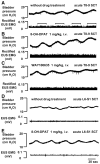
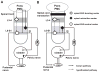
Lower urinary tract function is regulated by spinal and supraspinal reflexes that coordinate the activity of the urinary bladder and external urethral sphincter (EUS). Two types of EUS activity (tonic and bursting) have been identified in rats. This study in urethane-anesthetized female rats used cystometry, EUS electromyography, spinal cord transection (SCT) at different segmental levels, and analysis of the effects of 5-HT(1A) receptor agonist (8-OH-DPAT) and antagonist (WAY100635) drugs to examine the origin of tonic and bursting EUS activity. EUS activity was elicited by bladder distension or electrical stimulation of afferent axons in the pelvic nerve (pelvic-EUS reflex). Tonic activity evoked by bladder distension was detected in spinal cord-intact rats and after acute and chronic T8-9 or L3-4 SCT but was abolished after L6-S1 SCT. Bursting activity was abolished by all types of SCT except chronic T8-9 transection. 8-OH-DPAT enhanced tonic activity, and WAY100635 reversed the effect of 8-OH-DPAT. The pelvic-EUS reflex consisted of an early response (ER) and late response (LR) when the bladder was distended in spinal cord-intact rats. ER remained after acute or chronic T8-9 and L3-4 SCT, but was absent after L6-S1 SCT. LR occurred only in chronic T8-9 SCT rats where it was enhanced or unmasked by 8-OH-DPAT. The results indicate that spinal serotonergic mechanisms facilitate tonic and bursting EUS activity. The circuitry for generating different patterns of EUS activity appears to be located in different segments of the spinal cord: tonic activity at L6-S1 and bursting activity between T8-9 and L3-4.
Figures




References
-
- Chang HY, Cheng CL, Chen JJ, de Groat WC. Abstract Viewer (Online). Program No. 541.13. Society for Neuroscience; 2004. Role of glutamatergic and serotonergic mechanisms in urethral sphincter reflexes in urethane-anesthetized rats. http://www.sfn.org.
-
- Chang HY, Cheng CL, Chen JJ, Negoita FA, de Groat WC. Abstract Viewer (Online). Program No. 48.17. Society for Neuroscience; 2005. Influence of serotonergic mechanisms in the spinal cord on external urethral sphincter function during voiding. http://www.sfn.org.
-
- Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol. 2004;187:445–454. - PubMed
-
- Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995;678:40–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

