beta-Amyloid degradation and Alzheimer's disease
- PMID: 17047308
- PMCID: PMC1559921
- DOI: 10.1155/JBB/2006/58406
beta-Amyloid degradation and Alzheimer's disease
Abstract
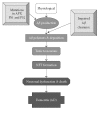
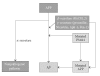
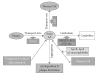
Extensive beta-amyloid (A beta) deposits in brain parenchyma in the form of senile plaques and in blood vessels in the form of amyloid angiopathy are pathological hallmarks of Alzheimer's disease (AD). The mechanisms underlying A beta deposition remain unclear. Major efforts have focused on A beta production, but there is little to suggest that increased production of A beta plays a role in A beta deposition, except for rare familial forms of AD. Thus, other mechanisms must be involved in the accumulation of A beta in AD. Recent data shows that impaired clearance may play an important role in A beta accumulation in the pathogenesis of AD. This review focuses on our current knowledge of A beta-degrading enzymes, including neprilysin (NEP), endothelin-converting enzyme (ECE), insulin-degrading enzyme (IDE), angiotensin-converting enzyme (ACE), and the plasmin/uPA/tPA system as they relate to amyloid deposition in AD.
Figures
References
-
- Zlokovic BV. Clearing amyloid through the blood-brain barrier. Journal of Neurochemistry. 2004;89(4):807–811. - PubMed
-
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends in Neurosciences. 2005;28(4):202–208. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochemical and Biophysical Research Communications. 1984;120(3):885–890. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

