Viral infection of the lungs through the eye
- PMID: 17050596
- PMCID: PMC1797451
- DOI: 10.1128/JVI.01437-06
Viral infection of the lungs through the eye
Abstract
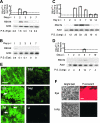
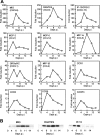
Respiratory syncytial virus (RSV) is the foremost respiratory pathogen in newborns and claims millions of lives annually. However, there has been no methodical study of the pathway(s) of entry of RSV or its interaction with nonrespiratory tissues. We and others have recently established a significant association between allergic conjunctivitis and the presence of RSV in the eye. Here we adopt a BALB/c mouse model and demonstrate that when instilled in the live murine eye, RSV not only replicated robustly in the eye but also migrated to the lung and produced a respiratory disease that is indistinguishable from the standard, nasally acquired RSV disease. Ocularly applied synthetic anti-RSV small interfering RNA prevented infection of the eye as well as the lung. RSV infection of the eye activated a plethora of ocular cytokines and chemokines with profound relevance to inflammation of the eye. Anticytokine treatments in the eye reduced ocular inflammation but had no effect on viral growth in both eye and lung, demonstrating a role of the cytokine response in ocular pathology. These results establish the eye as a major gateway of respiratory infection and a respiratory virus as a bona fide eye pathogen, thus offering novel intervention and treatment options.
Figures


References
-
- Adamus, G., M. Manczak, and M. Machnicki. 2001. Expression of CC chemokines and their receptors in the eye in autoimmune anterior uveitis associated with EAE. Investig. Ophthalmol. Vis. Sci. 42:2894-2903. - PubMed
-
- Barik, S. 2004. Control of nonsegmented negative-strand RNA virus replication by siRNA. Virus Res. 102:27-35. - PubMed
-
- Barik, S. 2005. Silence of the transcripts: RNA interference in medicine. J. Mol. Med. 83:764-773. - PubMed
-
- Bitko, V., N. E. Garmon, T. Cao, B. Estrada, J. E. Oakes, R. N. Lausch, and S. Barik. 2004. Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection. BMC Microbiol. 4:28. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

