Glycoprotein L disruption reveals two functional forms of the murine gammaherpesvirus 68 glycoprotein H
- PMID: 17050601
- PMCID: PMC1797276
- DOI: 10.1128/JVI.01616-06
Glycoprotein L disruption reveals two functional forms of the murine gammaherpesvirus 68 glycoprotein H
Abstract
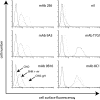
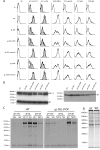
The herpesvirus glycoprotein H (gH) and gL associate to form a heterodimer that plays a central role in virus-driven membrane fusion. When archetypal alpha- or betaherpesviruses lack gL, gH misfolds and progeny virions are noninfectious. In order to define the role that gL plays in gamma-2 herpesvirus infections, we disrupted its coding sequence in murine gammaherpesvirus-68 (MHV-68). MHV-68 lacking gL folded gH into a conformation antigenically distinct from the form that normally predominates on infected cells. gL-deficient virions bound less well than the wild type to epithelial cells and fibroblasts. However, they still incorporated gH and remained infectious. The cell-to-cell spread of gL-deficient viruses was remarkably normal, as was infection, dissemination, and latency establishment in vivo. Viral membrane fusion was therefore gL independent. The major function of gL appeared to be allowing gH to participate in cell binding prior to membrane fusion. This function was most important for the entry of MHV-68 virions into fibroblasts and epithelial cells.
Figures







References
-
- Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. - PubMed
-
- Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. - PubMed
-
- Boname, J. M., B. D. de Lima, P. J. Lehner, and P. G. Stevenson. 2004. Viral degradation of the MHC class I peptide loading complex. Immunity 20:305-317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

