Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory
- PMID: 17050681
- PMCID: PMC1637622
- DOI: 10.1073/pnas.0607822103
Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory
Abstract
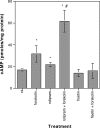
Small molecules that activate signaling pathways used by neurotrophic factors could be useful for treating CNS disorders. Here we show that the flavonoid fisetin activates ERK and induces cAMP response element-binding protein (CREB) phosphorylation in rat hippocampal slices, facilitates long-term potentiation in rat hippocampal slices, and enhances object recognition in mice. Together, these data demonstrate that the natural product fisetin can facilitate long-term memory, and therefore it may be useful for treating patients with memory disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. BioDrugs. 2005;19:97–127. - PubMed
-
- Fumagalli F, Racagni G, Riva MA. Pharmacogen J. 2006;6:95–104. - PubMed
-
- Sagara Y, Vahnnasy J, Maher P. J Neurochem. 2004;90:1144–1155. - PubMed
-
- Ishige K, Schubert D, Sagara Y. Free Radic Biol Med. 2001;30:433–446. - PubMed
-
- Adams JP, Sweatt JD. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

