Monitoring intermediate filament assembly by small-angle x-ray scattering reveals the molecular architecture of assembly intermediates
- PMID: 17050693
- PMCID: PMC1637561
- DOI: 10.1073/pnas.0603629103
Monitoring intermediate filament assembly by small-angle x-ray scattering reveals the molecular architecture of assembly intermediates
Abstract
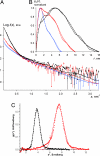
Intermediate filaments (IFs), along with microtubules, microfilaments, and associated cross-bridging proteins, constitute the cytoskeleton of metazoan cells. While crystallographic data on the dimer representing the elementary IF "building block" have recently become available, little structural detail is known about both the mature IF architecture and its assembly pathway. Here, we have applied solution small-angle x-ray scattering to investigate the in vitro assembly of a 53-kDa human IF protein vimentin at pH 8.4 by systematically varying the ionic strength conditions, and complemented these experiments by electron microscopy and analytical ultracentrifugation. While a vimentin solution in 5 mM Tris.HCl (pH 8.4) contains predominantly tetramers, addition of 20 mM NaCl induces further lateral assembly evidenced by the shift of the sedimentation coefficient and yields a distinct octameric intermediate. Four octamers eventually associate into unit-length filaments (ULFs) that anneal longitudinally. Based on the small-angle x-ray scattering experiments supplemented by crystallographic data and additional structural constraints, 3D molecular models of the vimentin tetramer, octamer, and ULF were constructed. Within each of the three oligomers, the adjacent dimers are aligned exclusively in an approximately half-staggered antiparallel A(11) mode with a distance of 3.2-3.4 nm between their axes. The ULF appears to be a dynamic and a relatively loosely packed structure with a roughly even mass distribution over its cross-section.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

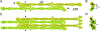

References
-
- Herrmann H, Aebi U. Curr Opin Cell Biol. 2000;12:79–90. - PubMed
-
- Parry DAD, Steinert PM. Intermediate Filament Structure. Heidelberg: Springer; 1995.
-
- Fuchs E, Weber K. Annu Rev Biochem. 1994;63:345–382. - PubMed
-
- Herrmann H, Hesse M, Reichenzeller M, Aebi U, Magin TM. Int Rev Cytol. 2003;223:83–175. - PubMed
-
- Herrmann H, Aebi U. Annu Rev Biochem. 2004;73:749–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

