Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli
- PMID: 17050704
- PMCID: PMC6674763
- DOI: 10.1523/JNEUROSCI.2305-06.2006
Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli
Abstract
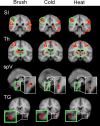
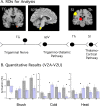
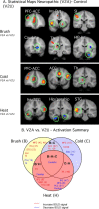
Functional magnetic resonance imaging was used to study patients with chronic neuropathic pain involving the maxillary region (V2) of the trigeminal nerve in patients with spontaneous pain and evoked pain to brush (allodynia). Patients underwent two functional scans (2-3 months apart) with mechanical and thermal stimuli applied to the affected region of V2 and to the mirror site in the unaffected contralateral V2 region, as well as bilaterally to the mandibular (V3) division. Patients were stimulated with brush, noxious cold, and noxious heat. Significant changes were observed in regions within and outside the primary trigeminal sensory pathway. Stimulation to the affected (neuropathic) side resulted in predominantly frontal region and basal ganglia activation compared with the control side. The differences were consistent with the allodynia to brush and cold. A region of interest-based analysis of the trigeminal sensory pathway revealed patterns of activation that differentiated between the affected and unaffected sides and that were particular to each stimulus. Activation in the spinal trigeminal nucleus was constant in location for all pain stimuli. Activation in other brainstem nuclei also showed differences in the blood oxygenation level-dependent signal for the affected versus the unaffected side. Thus, sensory processing in patients with trigeminal neuropathic pain is associated with distinct activation patterns consistent with sensitization within and outside of the primary sensory pathway.
Figures




References
-
- Afifi AK. The basal ganglia: a neural network with more than motor function. Semin Pediatr Neurol. 2003;10:3–10. - PubMed
-
- Aharon I, Becerraa L, Chabris CF, Borsook D. Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci Lett. 2006;392:159–164. - PubMed
-
- Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122:223–234. - PubMed
-
- Barker RA. The basal ganglia and pain. Int J Neurosci. 1988;41:29–34. - PubMed
-
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
