Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa
- PMID: 17050922
- PMCID: PMC1636254
- DOI: 10.1128/JB.00779-06
Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa
Abstract
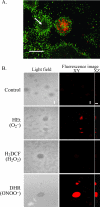
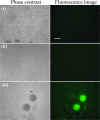
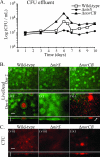
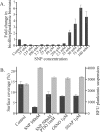
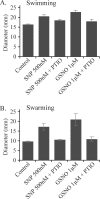
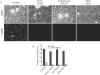
Bacterial biofilms at times undergo regulated and coordinated dispersal events where sessile biofilm cells convert to free-swimming, planktonic bacteria. In the opportunistic pathogen Pseudomonas aeruginosa, we previously observed that dispersal occurs concurrently with three interrelated processes within mature biofilms: (i) production of oxidative or nitrosative stress-inducing molecules inside biofilm structures, (ii) bacteriophage induction, and (iii) cell lysis. Here we examine whether specific reactive oxygen or nitrogen intermediates play a role in cell dispersal from P. aeruginosa biofilms. We demonstrate the involvement of anaerobic respiration processes in P. aeruginosa biofilm dispersal and show that nitric oxide (NO), used widely as a signaling molecule in biological systems, causes dispersal of P. aeruginosa biofilm bacteria. Dispersal was induced with low, sublethal concentrations (25 to 500 nM) of the NO donor sodium nitroprusside (SNP). Moreover, a P. aeruginosa mutant lacking the only enzyme capable of generating metabolic NO through anaerobic respiration (nitrite reductase, DeltanirS) did not disperse, whereas a NO reductase mutant (DeltanorCB) exhibited greatly enhanced dispersal. Strategies to induce biofilm dispersal are of interest due to their potential to prevent biofilms and biofilm-related infections. We observed that exposure to SNP (500 nM) greatly enhanced the efficacy of antimicrobial compounds (tobramycin, hydrogen peroxide, and sodium dodecyl sulfate) in the removal of established P. aeruginosa biofilms from a glass surface. Combined exposure to both NO and antimicrobial agents may therefore offer a novel strategy to control preestablished, persistent P. aeruginosa biofilms and biofilm-related infections.
Figures
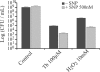
Comment in
-
When the party is over: a signal for dispersal of Pseudomonas aeruginosa biofilms.J Bacteriol. 2006 Nov;188(21):7325-7. doi: 10.1128/JB.01317-06. J Bacteriol. 2006. PMID: 17050919 Free PMC article. No abstract available.
References
-
- Aravind, L., V. Anantharaman, and L. M. Iyer. 2003. Evolutionary connections between bacterial and eukaryotic signaling systems: a genomic perspective. Curr. Opin. Microbiol. 6:490-497. - PubMed
-
- Beckman, J. S., and W. H. Koppenol. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271:C1424-C1437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

