The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-gamma
- PMID: 17052246
- PMCID: PMC2265875
- DOI: 10.1111/j.1365-2567.2006.02486.x
The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires interferon-gamma
Abstract
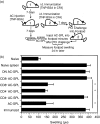
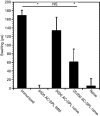
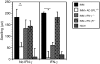
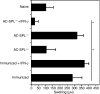
CD8(+) regulatory (suppressor) T cells are induced by complex cellular pathways in the spleens of mice that have received an injection of antigen into the anterior chamber (AC) of an eye, an immune-privileged site. Although these CD8(+) regulatory T cells perform an antigen-specific regulatory function for an immune response to self and non-self antigens, the mechanisms of the activation or function of these regulatory cells are not clear. Here, we describe a novel mechanism for the activation of splenic CD8(+) regulatory T cells induced by injection of antigen into the AC. Immunization of mice with trinitrophenyl and bovine serum albumin (TNP-BSA) amplified AC-induced splenic CD8(+) regulatory T cells that suppressed the initiation of contact sensitivity when transferred to immunized, challenged mice. These CD8(+) regulatory T cells were produced independently of perforin, indicating that they are not canonical cytotoxic T cells. Fas ligand (FasL)-deficient CD8(+) regulatory T-cell function was rescued by inclusion of exogenous interferon-gamma (IFN-gamma), demonstrating that the expression of FasL by CD8(+) regulatory T cells was dispensable, but IFN-gamma was not. Ultimately, we demonstrated that the generation of these CD8(+) regulatory T cells occurred independently of IFN-gamma, but their suppressor function required IFN-gamma receptor stimulation.
Figures


Similar articles
-
Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the antigen-induced production of immunoglobulin G1 antibodies.Immunology. 2004 Sep;113(1):44-56. doi: 10.1111/j.1365-2567.2004.01928.x. Immunology. 2004. PMID: 15312135 Free PMC article.
-
The induction of splenic suppressor T cells through an immune-privileged site requires an intact sympathetic nervous system.J Neuroimmunol. 2004 Aug;153(1-2):40-9. doi: 10.1016/j.jneuroim.2004.04.008. J Neuroimmunol. 2004. PMID: 15265662
-
Intracameral injection of antigen potentiates the production of antigen-specific T cell proteins in serum after the induction of delayed-type hypersensitivity.Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1470-6. Invest Ophthalmol Vis Sci. 1995. PMID: 7775125
-
Control of delayed-type hypersensitivity by ocular- induced CD8+ regulatory t cells.Chem Immunol Allergy. 2008;94:138-149. doi: 10.1159/000154998. Chem Immunol Allergy. 2008. PMID: 18802344 Review.
-
Ocular immunosuppressive microenvironment.Chem Immunol. 1999;73:72-89. doi: 10.1159/000058738. Chem Immunol. 1999. PMID: 10590575 Review. No abstract available.
Cited by
-
Alloimmunity and Tolerance in Corneal Transplantation.J Immunol. 2016 May 15;196(10):3983-91. doi: 10.4049/jimmunol.1600251. J Immunol. 2016. PMID: 27183635 Free PMC article. Review.
-
Two different regulatory T cell populations that promote corneal allograft survival.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6566-74. doi: 10.1167/iovs.10-6161. Epub 2010 Aug 11. Invest Ophthalmol Vis Sci. 2010. PMID: 20702818 Free PMC article.
-
Upregulation of CD94 on CD8+T cells in anterior chamber-associated immune deviation.BMC Immunol. 2008 Sep 25;9:53. doi: 10.1186/1471-2172-9-53. BMC Immunol. 2008. PMID: 18816417 Free PMC article.
-
Advances on Non-CD4 + Foxp3+ T Regulatory Cells: CD8+, Type 1, and Double Negative T Regulatory Cells in Organ Transplantation.Transplantation. 2015 Aug;99(8):1553-9. doi: 10.1097/TP.0000000000000813. Transplantation. 2015. PMID: 26193065 Free PMC article. Review.
-
Influence of CD8+ T regulatory cells on intraocular tumor development.Front Immunol. 2012 Sep 28;3:303. doi: 10.3389/fimmu.2012.00303. eCollection 2012. Front Immunol. 2012. PMID: 23060881 Free PMC article.
References
-
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;14:535–62. - PubMed
-
- Laloux V, Beaudoin L, Jeske D, Carnaud C, Lehuen A. NK T cell-induced protection against diabetes in Va 14-Ja281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J Immunol. 2001;166:3749–56. - PubMed
-
- Wang Y, Goldschneider I, O'Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–6. - PubMed
-
- Sakaguchi S, Sakaguchi NM, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

