A system for rating the stability and strength of medical evidence
- PMID: 17052350
- PMCID: PMC1624842
- DOI: 10.1186/1471-2288-6-52
A system for rating the stability and strength of medical evidence
Abstract
Background: Methods for describing one's confidence in the available evidence are useful for end-users of evidence reviews. Analysts inevitably make judgments about the quality, quantity consistency, robustness, and magnitude of effects observed in the studies identified. The subjectivity of these judgments in several areas underscores the need for transparency in judgments.
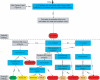
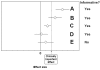
Discussion: This paper introduces a new system for rating medical evidence. The system requires explicit judgments and provides explicit rules for balancing these judgments. Unlike other systems for rating the strength of evidence, our system draws a distinction between two types of conclusions: quantitative and qualitative. A quantitative conclusion addresses the question, "How well does it work?", whereas a qualitative conclusion addresses the question, "Does it work?" In our system, quantitative conclusions are tied to stability ratings, and qualitative conclusions are tied to strength ratings. Our system emphasizes extensive a priori criteria for judgments to reduce the potential for bias. Further, the system makes explicit the impact of heterogeneity testing, meta-analysis, and sensitivity analyses on evidence ratings. This article provides details of our system, including graphical depictions of how the numerous judgments that an analyst makes can be combined. We also describe two worked examples of how the system can be applied to both interventional and diagnostic technologies.
Summary: Although explicit judgments and formal combination rules are two important steps on the path to a comprehensive system for rating medical evidence, many additional steps must also be taken. Foremost among these are the distinction between quantitative and qualitative conclusions, an extensive set of a priori criteria for making judgments, and the direct impact of analytic results on evidence ratings. These attributes form the basis for a logically consistent system that can improve the usefulness of evidence reviews.
Figures






References
-
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr, Zaza S. Grading quality of evidence and strength of recommendations. BMJ. 328:1490. http://bmj.bmjjournals.com/cgi/reprint/328/7454/1490 2004 Jun 19; - PMC - PubMed
-
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O'Connell D, Oxman AD, Phillips B, Schunemann H, Edejer TT, Vist GE, Williams JW, Jr, GRADE Working Group Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 4:38. doi: 10.1186/1472-6963-4-38. 2004 Dec 22; - DOI - PMC - PubMed
-
- Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, Hill S, Jaeschke R, Liberati A, Magrini N, Mason J, O'Connell D, Oxman AD, Phillips B, Schunemann H, Edejer TT, Vist GE, Williams JW, Jr, GRADE Working Group Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res. 5:25. doi: 10.1186/1472-6963-5-25. 2005 Mar 23; - DOI - PMC - PubMed
-
- Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schunemann H. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129:174–81. doi: 10.1378/chest.129.1.174. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

