Two functional modes of a nuclear receptor-recruited arginine methyltransferase in transcriptional activation
- PMID: 17052457
- PMCID: PMC1647399
- DOI: 10.1016/j.molcel.2006.09.020
Two functional modes of a nuclear receptor-recruited arginine methyltransferase in transcriptional activation
Abstract
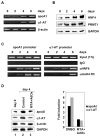
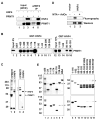
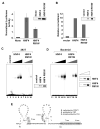
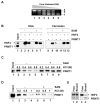
Nuclear receptors, like other transcriptional activators, switch on gene transcription by recruiting a complex network of coregulatory proteins. Here, we have identified the arginine methyltransferase PRMT1 as a coactivator for HNF4, an orphan nuclear receptor that regulates the expression of genes involved in diverse metabolic pathways. Remarkably, PRMT1, whose methylation activity on histone H4 strongly correlates with induction of HNF4 target genes in differentiating enterocytes, regulates HNF4 activity through a bipartite mechanism. First, PRMT1 binds and methylates the HNF4 DNA-binding domain (DBD), thereby enhancing the affinity of HNF4 for its binding site. Second, PRMT1 is recruited to the HNF4 ligand-binding domain (LBD) through a mechanism that involves the p160 family of coactivators and methylates histone H4 at arginine 3. This, together with recruitment of the histone acetyltransferase p300, leads to nucleosomal alterations and subsequent RNA polymerase II preinitiation complex formation.
Figures


References
-
- An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–748. - PubMed
-
- An W, Roeder RG. Reconstitution and transcriptional analysis of chromatin in vitro. Methods Enzymol. 2004;377:460–474. - PubMed
-
- Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18:263–272. - PubMed
-
- Chen D, Huang SM, Stallcup MR. Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J Biol Chem. 2000;275:40810–40816. - PubMed
-
- Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, Darnell JE., Jr Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466–2477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

