Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane
- PMID: 17053074
- PMCID: PMC1637610
- DOI: 10.1073/pnas.0605835103
Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane
Abstract
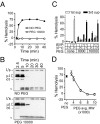
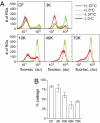
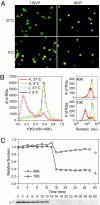
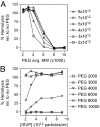
During cell entry, reovirus particles with a diameter of 70-80 nm must penetrate the cellular membrane to access the cytoplasm. The mechanism of penetration, without benefit of membrane fusion, is not well characterized for any such nonenveloped animal virus. Lysis of RBCs is an in vitro assay for the membrane perforation activity of reovirus; however, the mechanism of lysis has been unknown. In this report, osmotic-protection experiments using PEGs of different sizes revealed that reovirus-induced lysis of RBCs occurs osmotically, after formation of small size-selective lesions or "pores." Consistent results were obtained by monitoring leakage of fluorophore-tagged dextrans from the interior of resealed RBC ghosts. Gradient fractionations showed that whole virus particles, as well as the myristoylated fragment mu1N that is released from particles, are recruited to RBC membranes in association with pore formation. We propose that formation of small pores is a discrete, intermediate step in the reovirus membrane-penetration pathway, which may be shared by other nonenveloped animal viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

