Zotepine for schizophrenia
- PMID: 17054149
- PMCID: PMC7017977
- DOI: 10.1002/14651858.CD001948.pub2
Zotepine for schizophrenia
Abstract
Background: Zotepine is a relatively new antipsychotic often used for the treatment of people with schizophrenia. It is claimed to be particularly effective for negative symptoms.
Objectives: To determine the effects of zotepine compared with placebo, typical and other atypical antipsychotic drugs for schizophrenia and related psychoses.
Search strategy: For the 2006 update we searched the Cochrane Schizophrenia Group's register of trials.
Selection criteria: We included all randomised clinical trials comparing zotepine with other treatments for people with schizophrenia or other psychoses.
Data collection and analysis: We independently inspected citations and abstracts, ordered papers, re-inspected these and assessed their quality. For homogenous dichotomous data we calculated the relative risk (RR), 95% confidence intervals (CI) and, where appropriate, numbers needed to treat/harm (NNT/H) on an intention-to-treat basis. For continuous data, we calculated weighted mean differences (WMD). We inspected all data for heterogeneity.
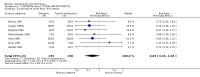
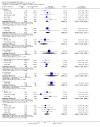
Main results: The review currently includes 11 studies with 966 participants. Most outcomes were short term (4-12 weeks). We found no data for outcomes such as relapse, time in hospital, satisfaction with care and day-to-day functioning. Compared with placebo, mental state ratings favoured zotepine (n=106, 1 RCT, RR No 20% decrease in BPRS 0.44 CI 0.3 to 0.7, NNT 3 CI 2 to 6) using the last observation carried forward method. For the comparison with typical drugs, limited data suggest that zotepine may be as effective as these older medications. Mental state measures of 'no clinically important improvement' favour zotepine when compared with other active drugs (n=356, 4 RCTs, RR 0.77 CI 0.7 to 0.9, NNT 7 CI 4 to 22). About one third of people in both the zotepine and control groups left the studies before trial completion. Zotepine may result in less movement disorder adverse effects than typical antipsychotic drugs. Trials have not highlighted clear differences between zotepine and other atypical drugs.
Authors' conclusions: Zotepine may be a valuable addition to the class of atypical antipsychotic drugs. However, more data from existing studies is urgently needed to increase confidence in the findings of this review. In addition to this, new data from well planned, conducted and reported long term pragmatic randomised trials are needed. Otherwise clinical use of zotepine will be based upon speculation of short explanatory trials for everyday practice.
Conflict of interest statement
Mark Fenton has lead Jansen, Lilly and Zeneca sponsored workshops for clinicians.
Prasanna De‐Silva has received financial assistance to attend various scientific meetings and conferences world‐wide.
Stephen Cooper has undertaken trials of zotepine with employees of Knoll Pharmaceuticals.
The Cochrane Schizophrenia Group has received general support funding from Eli Lilly during the years 1996‐8 (see Group Module). This, along with some funds from other pharmaceutical companies, is used to support any ongoing work of the editorial base and is not linked to any particular review (annual report available on request).
Figures













































Update of
-
Zotepine for schizophrenia.Cochrane Database Syst Rev. 2000;(2):CD001948. doi: 10.1002/14651858.CD001948. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2006 Oct 18;(4):CD001948. doi: 10.1002/14651858.CD001948.pub2. PMID: 10796671 Updated.
References
References to studies included in this review
Barnas 1987 {published data only}
-
- Barnas C, Stuppack CH, Miller C, Haring C, Sperner‐Unterweger B, Fleischhacker WW. Zotepine in the treatment of schizophrenic patients with prevailingly negative symptoms: A double blind trial vs. haloperidol. International Clinical Psychopharmacology 1992;7:23‐7. - PubMed
-
- Barnas C, Stuppack CH, Miller C, Haring C, Sperner‐Unterweger B, Fleischhacker WW. Zotepine: treatment of schizophrenic patients with predominantly negative symptoms. A double‐blind study vs. haloperidol] [Zotepin: Die Behandlung schizophrener Patienten mit vorherrschender Negativsymptomatik. Eine Doppelblindstudie vs. Haloperidol]. Fortschritte der Neurologie Psychiatrie 1991;59:36‐40. - PubMed
Cooper 1999a {unpublished data only}
-
- Cooper SJ, Butler A, Tweed J, Welch CP. Zotepine in the treatment of chronic schizophrenia. 10 th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
-
- Cooper SJ, Raniwalla J, Welch C. Zotepine in acute exacerbation of schizophrenia: a comparison versus chlorpromazine and placebo. XX th Collegium Internationale Neuro‐psychopharmacologicum, Melbourne, Australia. 1996.
-
- Cooper SJ, Tweed J, Raniwalla J, Butler A, Welch C. A placebo controlled comparison of zotepine versus chlorpromazine in patients with acute exacerbation of schizophrenia. Acta Psychiatrica Scandinavica 2000;101(3):218‐25. - PubMed
-
- Cooper SJ, Tweed J, Raniwalla J, Welch C. A placebo‐controlled comparison of zotepine versus chlorpromazine in patients with acute exacerbation of schizophrenia. Unpublished manuscript 1999. - PubMed
-
- Cooper SJ, Welch CP. A Comparison of zotepine and chlorpromazine on BPRS sub‐scores. 10 th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. 1997.
Cooper 1999b {unpublished data only}
-
- Cooper SJ, Butler A, Tweed J, Raniwalla J, Welch C. Zotepine in the prevention of relapse. Biological Psychiatry 1997;42:41S.
-
- Cooper SJ, Butler A, Tweed J, Raniwalla J, Welch C. Zotepine in the prevention of relapse. Sixth World Congress of Biological Psychiatry, Nice, France. 1997.
-
- Cooper SJ, Butler A, Tweed J, Welch C, Raniwalla J. Zotepine in the prevention of reoccurrence: a randomised, double blind, placebo controlled study for chronic schizophrenia. Unpublished manuscript 1999. - PubMed
-
- Cooper SJ, Butler A, Tweed JA, Welch CP, Wighton AJ, Appleby P, Reynolds CW, Ruck A. Zotepine is effective in preventing recurrence in patients with chronic schizophrenia. 11 th European College of Neuropsychopharmacology Congress Oct 31 ‐ Nov 4, Paris, France. 1998.
-
- Cooper SJ, Butler A, Tweed JA, Welch CP, Wighton AJ, Appleby P, Reynolds CW, Ruck A. Zotepine is effective in preventing recurrence in patients with chronic schizophrenia. Schizophrenia Research 2000;41(1):207‐8.
Dieterle 1991 {published data only}
-
- Dieterle DM, Muller Spahn F, Ackenheil M. Effectiveness and tolerance of zotepine in a double‐blind comparison with perazine in schizophrenic patients [Wirksamkeit und vertraglichkeit von zotepin im doppelblindvergleich mit perazin bei schizophrenen patienten]. Fortschritte der Neurologie Psychiatrie 1991;59:18‐22. - PubMed
-
- Muller Spahn F, Dieterle D, Ackenheil M. Clinical effectiveness of zotepine in treatment of negative schizophrenic symptoms. Results of an open and a double‐blind controlled trial [Klinische wirksamkeit von zotepin in der behandlung schizophrener minussymptomatik. Ergebnisse einer offenen und einer doppelblind‐ kontrollierten studie]. Fortschritte der Neurologie Psychiatrie 1991;59:30‐5. - PubMed
Fleischhacker 1989 {published data only}
-
- Fleischhacker WW, Barnas C, Stuppack CH, Sperner‐Unterweger B, Miller C, Hinterhuber H. Zotepine vs. haloperidol in paranoid schizophrenia: a double‐blind study [Zotepin vs. haloperidol bei paranoider schizophrenie: eine doppelblindstudie]. Fortschritte der Neurologie Psychiatrie 1991;59:10‐30. - PubMed
-
- Fleischhacker WW, Barnas C, Stuppack CH, Unterweger B, Miller C, Hinterhuber H. Zotepine vs. haloperidol in paranoid schizophrenia: a double‐blind trial. Psychopharmacology Bulletin 1989;25:97‐100. - PubMed
-
- Stuppäck H, Barnas B, Unterweger B, Fleischhacker WW. Zotepine vs. Haloperidol in acute schizophrenia: a double blind trial. Schizophrenia Research 1998;1(2‐3):200‐1.
Klieser 1996 {published data only}
-
- Klieser E, Lehmann E, Heinrich K. Risperidone in Comparison with various treatments of schizophrenia. In: Kane JM, Möller HJ editor(s). Medical Psychiatry. Vol. 3, New York: Marcel Dekker, 1996.
-
- Klieser E, Lehmann E, Tegeler J. Double‐blind comparison of 3 x 75 mg zotepine und 3 x 4 mg haloperidol in acute schizophrenic patients [Doppelblindvergleich von 3 x 75 mg zotepin und 3 x 4 mg haloperidol bei akut schizophrenen patienten]. Fortschritte der Neurologie Psychiatrie 1991;59:14‐7. - PubMed
Meyer‐Lindberg 1996 {published data only}
-
- Gallhofer B, Gruppe H, Bauer U. Zotepine versus clozapine: A comparison of the impact on the cognitive dysfunction syndrome in schizophrenia (a double blind trial). Pharmacopsychiatry 1995;28:179. [11th Congress of the European College of Neuropsychopharmacology [CD‐ROM]: Conifer, Excerpta Medica Medical Communications BV, 1998 P6037]
-
- Meyer‐Lindenberg A, Gruppe H, Bauer U, Lis S, Krieger S, Gallhofer B. Improvement of cognitive function in schizophrenic patients receiving clozapine or zotepine: results from a double‐blind study. Pharmacopsychiatry 1997;30:35‐42. - PubMed
Moller 2004 {published data only}
-
- Moller HJ, Riedel M, Muller N, Fischer W, Kohnen R. Zotepine versus placebo in the treatment of schizophrenic patients with stable primary negative symptoms: a randomized double‐blind multicenter trial. Pharmacopsychiatry 2004;37(6):270‐8. [MEDLINE: ; EMBASE: 2004519196] - PubMed
Petit 1996 {published data only}
-
- Petit M, Raniwalla J, Leutenegger E, Tweed J, Kelly F. Double‐blind study of zotepine vs haloperidol in schizophrenia. 8th European College of Neuropsychopharmacology Congress; 1995 Sep 30 ‐ Oct 4; Venice, Italy. 1995.
-
- Petit M, Raniwalla J, Tweed J, Leutenegger E, Dollfus S, Kelly F. A comparison of an atypical and typical antipsychotic, zotepine versus haloperidol in patients with acute exacerbation of schizophrenia: a parallel‐group double‐blind trial. Psychopharmacology Bulletin 1996;32:81‐7. - PubMed
Sarai 1987 {published data only}
-
- Sarai K, Okada M. Comparison of efficacy of zotepine and thiothixene in schizophrenia in a double‐blind study. Pharmacopsychiatry 1987;20:38‐46. - PubMed
Wetzel 1991 {published data only}
-
- Wetzel H, Bardeleben U, Holsboer F, Benkert O. Zotepine versus perazine in patients with paranoid schizophrenia: a double‐blind controlled trial of its effectiveness [Zotepin versus perazin bei patienten mit paranoider schizophrenie: eine doppelblind‐kontrollierte wirksamkeitsprufung]. Fortschritte der Neurologie Psychiatrie 1991;59:23‐9. - PubMed
References to studies excluded from this review
Faulkner 1997 {published data only}
-
- Faulkner RD, Hinson J, Leone MB, Moult J, Ghani S, Velagapudi RB. Effect of food on the pharmacokinetics of zotepine and norzotepine in healthy subjects. 10th ECNP (European College of Neuropsychopharmacology) Congress, Vienna, Austria. Sep 13 ‐ 17, 1997.
Fleischhacker 1998 {published data only}
-
- Fleischhacker WW. Problems in evaluating new antipsychotic drugs [Zur problematik der beurteilung neuer antipsychotika]. Wiener Medizinische Wochenschrift 1998;148:266‐72. - PubMed
Fukahori 1995 {published data only}
-
- Fukahori M, Inanaga K, Suetsugu M. Clinical evaluation of sultopride hydrochloride on outpatients with schizophrenia. Japanese Pharmacology and Therapeutics 1995;23:267‐80.
Hada 1985 {published data only}
-
- Hada Y. Clinical effects of zotepine in outpatients with schizophrenia. Shinryo to Shin'yaku 1985;22(2):435‐44.
Harada 1991 {published data only}
-
- Harada T, Otsuki S, Fujiwara Y. Effectiveness of zotepine in therapy‐refractory psychoses. An open, multicenter study in eight psychiatric clinics [Wirksamkeit von ztepin bei therapieresistenten pychosen. Eine offene, multizentrische sudie in acht psychiatrischen kiniken]. Fortschritte der Neurologie Psychiatrie 1991;59:41‐4. - PubMed
-
- Harada T, Sato M, Omori B, Hirata J, Fujimoto A, Nishioka H, Takahashi Y, Oshimo T, Suemaru K. Effectiveness of zotepine in refractory psychoses. Shin'yaku to Rinsho 1987;36(9):1447‐59.
Hen 1987 {published data only}
-
- Hen LS. Controlled clinical trial of zotepine in schizophrenia. Chinese Journal of Nervous and Mental Disorders 1987;1392:87.
Komada 1987 {published data only}
-
- Komada A, Kishimoto A, Tanaka K, Fukuma E, Kunimoto N. Effects of zotepine in acute treatment and maintenance therapy of schizophrenic out‐patients. Igaku to Yakugaku 1987;18(4):1175‐87.
Naber 1997 {published data only}
-
- Briken P, Nika E, Moritz S, Haasen C, Perro C, Yagdiran O, Naber D, Krausz M. Effect of zotepine, olanzapine and rispiridone on hostility in schizophrenic patients. Schizophrenia Research 2002;57:311‐13. - PubMed
-
- Naber D. Evidence of efficacy of neuroleptics in effective versus negative symptoms. 10th European College of Neuropsychopharmacology Congress (ECNP ), Vienna, Austria. September 13‐17, 1997.
Needham 1996 {published data only}
-
- Needham PL, Atkinson J, Skill M J, Heal DJ. Zotepine: preclinical tests predict antipsychotic efficacy and an atypical profile. Psychopharmacology Bulletin 1996;32:123‐8. - PubMed
Nishizono 1994 {published data only}
-
- Nishizono M. A comparative trial of zotepine, chlorpromazine and haloperidol in schizophrenic patients. Shinkei Seishin Yakuri 1994;10(2):30.
Ohhara 1988 {published data only}
-
- Ohhara K, Suzuki Y, Kawaguchi K, et al. Effects of zotepine on chronic schizophrenics with a mean disease duration of 19.9 years. Shinryo to Shinyaku 1988;25(6):1205‐11.
Prakash 1998 {published data only}
-
- Prakash A, Lamb HM. Zotepine: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs 1998;9:153‐75.
Saletu 1987 {published data only}
-
- Saletu B, Grunberger J, Linzmayer L, Anderer P. Comparative placebo‐controlled pharmacodynamic studies with zotepine and clozapine utilizing pharmaco‐EEG and psychometry. Pharmacopsychiatry 1987;20:12‐27. - PubMed
Takashina 1986 {published data only}
-
- Takashina K, Yasuda T, Terada H, Yoshida H, Okuda J. Clinical use of zotepine (Lodopin) for schizophrenia and mania. Shinryo to Shin'yaku 1986;23(9):2043‐69.
Welch BPI1201‐USA {published data only}
-
- Welch CP, Kilpatrick AT, Butler A, Tweed JA. The efficacy of zotepine in reducing negative symptoms. Knoll Pharmacueticals Advertising Material 1999.
-
- Welch CP, Kilpatrick AT, Butler A, Tweed JA. The efficacy of zotepine in reducing negative symptoms. Knoll Pharmacueticals Advertising Material 1999.
References to studies awaiting assessment
Imai 1982 {published data only}
-
- Imai H, Ohtani W, Kondo H. Comparison of efficacy of zotepine and perphenazine in schizophrenia by double‐blind, controlled study. Japanese Journal Neuropsychopharmacology 1982;2:61.
Iwanaga 1980 {published data only}
-
- Owanaga M, Okada I, Ichinomiya Y. Clinical evaluation of zotepine in chronic schizophrenia. A multi‐institutional double‐blind study in comparison with propericiazine. Yakuri To Chiryo 1980;9:121.
Okada 1980 {published data only}
-
- Okada M, Sarai K, Hamano H. Comparison of efficacy of zotepine and tiotixene in schizophrenia by double‐blind study. Journal of Clinical Psychiatry 1980;22(9):947‐65.
Additional references
Adams 1999
-
- Adams CE, Gray R, Daniels J, Thornley B, Philpott H, Wahlbeck K. A survey of UK consultant psychiatrists first and second choice drug treatments for schizophrenia. Schizophrenia Trials Meeting, Stratford upon Avon. 5‐7th May, 1999.
Altman 1996
Andreasen 1984
-
- Andreasen NC. Scale for the assessment of negative symptoms (SANS). Iowa City: University of Iowa, 1984.
APA 1997
-
- American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 1997;154(4):1‐63. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement Improving the quality of reporting of randomized controlled trials. The CONSORT statement.. JAMA 1996;8:637‐9. - PubMed
Bland 1997
Buckley 1997
-
- Buckley PF. New dimensions in the pharmacologic treatment of schizophrenia and related psychoses. Journal of Clinical Pharmacology 1997;37:363‐78. - PubMed
Carpenter 1994
-
- Carpenter WT Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330:681‐90. - PubMed
Casey 1997
-
- Casey DE. The relationship of pharmacology to side effects. Journal of Clinical Psychiatry 1997;58(10):55‐62. - PubMed
Chalmers 1983
-
- Chalmers TC, Celano P, Sacks HS, Smith H Jr. Bias in treatment assignment in controlled clinical trials. New Englans Journal of Medicine 1983;309(22):1358‐61. - PubMed
COSTART 1990
-
- US Food, Drug Administration. COSTART coding sysmbols for thesaurus of adverse reaction terms. 2. Rockville, MD: Food and Drug Administration, 1990.
CWG 1998
-
- Collaborative Working Group on Clinical Trial Evaluations. Adverse effects of the atypical antipsychotics. Journal of Clinical Psychiatry 1998;59:17‐22. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Duggan 1999
-
- Duggan L, Fenton M, Dardennes RM, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. The Cochrane Library 1999, Issue 2.
Egger 1997
Erzigkeit 1986
-
- Erzigkeit H. Manual zum syndrom Kurztest (SKT). Vaterstetten: Vless‐Verlag, 1986.
Geddes 1999
-
- Geddes J. Clinical Guidelines. Schizophrenia Trials Meeting, Stratford‐upon‐Avon. 5‐7th May, 1999.
Glazer 1997
-
- Glazer WM, Johnstone BM. Pharmacoeconomic evaluation of antipsychotic therapy for schizophrenia. Journal of Clinical Psychiatry 1997;58(10):50‐4. - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Washington DC: National Institute of Mental Health, 1976:217‐22. [Publication No.76‐338]
Heres 2006
-
- Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why Olanzapine Beats Risperidone, Risperidone Beats Quetiapine, and Quetiapine Beats Olanzapine: An Exploratory Analysis of Head‐to‐Head Comparison Studies of Second‐Generation Antipsychotics. American Journal of Psychiatry 2006;163(2):185‐94. - PubMed
Higgins 2003
Higgins 2005
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: Higgins JPT, Green S editor(s). The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd., 2005.
Jadad 1996
-
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Jenkinson 1997
-
- Jenkinson C, Layte R, Lawrence K. Development and testing of the SF‐36 summary scale scores in the United Kingdom: Results from a large scale survey and a clinical trial. Medical Care 1997;35:410‐16. - PubMed
Kane 1996
-
- Kane JM. Factors which can make patients difficult to treat. British Journal of Psychiatry 1996;169(31):10‐4. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Kerwin 1994
-
- Kerwin RW. The new atypical antipsychotics. A lack of extrapyramidal side‐effects and new routes in schizophrenia research. British Journal of Psychiatry 1994;164:141‐8. - PubMed
Kerwin 1996
-
- Kerwin R, Taylor D. New antipsychotics. A review of their current status and clinical potential. CNS Drugs 1996;6:71‐82.
Meltzer 1996
-
- Meltzer HY. Cost‐effectiveness of clozapine treatment. Journal of Clinical Psychiatry ‐ Monograph Series 1996;14:16‐7.
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Montgomery 2004
-
- Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, Holland R. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled Clinical Trials 2004;25 (Dec)(6):598‐612. - PubMed
NIMH 1981
-
- National Institue of Mental Health. Dosage record and treatment emergent symptom scale (DOTES). Collegium Internationale Psychiatriae Scalarum: Internationale Skalen fur Psychiatrie. Weinheim: Beltz Test, 1981.
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Pantelis 1997
-
- Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW. Frontal‐striatal cognitive deficits in patients with chronic schizophrenia. Brain 1997;120(10):1823‐43. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Silverstone 1995
-
- Silverstone T, Turner P. Drug treatment in psychiatry. 5th Edition. Cornwall: TJ Press, 1995.
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. - PubMed
Simpson 1979
-
- Simpson GM, Lee JH, Zoubouk B, Gardos G. A rating scale for tardive dyskinesia. Psychopharmacology 1979;64:171‐79. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Wood Mackenzie 1998
-
- Wood Mackenzie. Review of the Global Schizophrenia Market. PharmaForum 1998;39:9.
www.psychotropics.dk
-
- Psychotropics. www.psychotropics.dk/default.asp.
References to other published versions of this review
Fenton 2000
-
- Fenton M, Morris S, De‐Silva P, Bagnall A, Cooper SJ, Gammelin G, Leitner M. Zotepine for schizophrenia. Cochrane Database Systematic Reviews 2000, Issue 2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

