Bicyclol for chronic hepatitis B
- PMID: 17054206
- PMCID: PMC12494191
- DOI: 10.1002/14651858.CD004480.pub2
Bicyclol for chronic hepatitis B
Abstract
Background: Bicyclol is a novel synthetic 'anti-hepatitis' drug, used in China for chronic hepatitis B. Until now, systematic reviews of bicyclol therapy have not been performed.
Objectives: To study the benefits and harms of bicyclol for patients with chronic hepatitis B.
Search strategy: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register (July 2005), The Cochrane Central Register of Controlled Trials in The Cochrane Library (Issue 2, 2005), MEDLINE (1950 to July 2005), EMBASE (1980 to July 2005), Science Citation Index Expanded (1945 to July 2005), The Chinese Biomedical Database (1994 to August 2005), VIP Chinese Science and Technique Journals Database (1994 to August 2005), and China National Infrastructure (CNKI)(1994 to August 2005). We also contacted manufacturers and researchers in the field.
Selection criteria: Randomised clinical trials with bicyclol versus no intervention, placebo, or other interventions were included, irrespective of blinding, publication status, or language.
Data collection and analysis: The primary outcome measures were mortality (total and liver-related) and liver-related morbidity (eg, cirrhosis and carcinoma). Secondary outcome measures were viral response and liver histology.
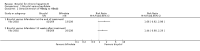
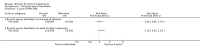
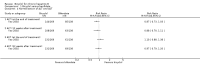
Main results: The search identified one randomised clinical trial comparing bicyclol with bifendate (biphenyldicarboxylate) for patients with hepatitis B. The follow-up was three months. There was no evidence that bicyclol was superior to bifendate for loss of HBeAg (RR 1.38, 95% CI 0.95 to 2.00), seroconversion of HBeAg to HBeAb (RR 1.44, 95% CI 0.90 to 2.29), loss of HBV DNA (RR 1.19, 95%CI 0.93 to 1.53), or number of patients with normalised alanine aminotransferase and aspartate aminotransferase activity (RR 0.88, 95% CI 0.70 to 1.11 and RR 0.97, 95% CI 0.79 to 1.20, respectively).
Authors' conclusions: Only one randomised clinical trial has examined the potential benefit of bicyclol for patients with chronic hepatitis B. This small, short-term trial found no evidence to support or refute its use. Large, randomised double-blind clinical trials with long-term follow-up are needed to examine the possible benefits and harms associated with bicyclol. Bicyclol can only be recommended for use in randomised trials.
Conflict of interest statement
None known.
Figures


Update of
- doi: 10.1002/14651858.CD004480
References
References to studies included in this review
Yao 2002 {published and unpublished data}
-
- Yao GB, Ji YY, Wang QH, Zhou XQ, Xu DZ, Chen XY, et al. A randomized double‐blind controlled trial of bicyclol in treatment of chronic hepatitis B. Chinese Journal of New Drug and Clinical Remedies 2002;21(8):457‐62.
References to studies excluded from this review
Feng 2004 {published data only}
-
- Feng JH, Wei H, Gao QM, Gao WJ, Wei M. Bicyclol in the treatment of hepatitis B. New Medicine 2004;35(4):219‐29.
Ji 2001 {published data only}
-
- Ji YY, Cheng NN, Yao GB. Pharmacokinetic study of bicyclol in thirty healthy volunteers. Chinese Journal of Clinical Pharmacology 2001;6(3):218‐21.
Pan 2004 {published data only}
-
- Pan YQ. Bicyclol in the treatment of 50 patients with hepatitis B. World Journal of Infection 2004;4(2):166.
Sen 2004 {published data only}
-
- Sen W, Wang XY, Tong LX, Xie CJ, Chen BL. Dynamic observation for combined treatment of chronic hepatitis B. Clinical Focus 2004;19(23):1359‐60.
Shen 2004 {published data only}
-
- Shen LR, Xiao LH, Xiao JL. Effect of bicyclol in the treatment of chronic hepatitis B. Journal of Jiangxi Medicine and Pharmocy 2004;39(2):137.
Shi 2002 {published data only}
-
- Shi WQ, Zhang Y. Clinical observation of bicyclol in the treatment of chronic hepatitis B. Infectious Diseases Information 2002;15(4):173.
Shi 2005 {published data only}
-
- Shi BB, Peng J, Wen FY, Hou JL. Effect of bicyclol tablets on cellular immune responses in patients with chronic hepatitis B. Di Yi Jun Yi Da Xue Xue Bao (Journal of The First Military Medical University) 2005;25(6):706‐8. - PubMed
Wang 2002 {published data only}
-
- Wang TD, Yang H, Gu JJ. Clinical observation of bicyclol in treating chronic hepatitis B in 60 cases. Chinese Clinical Drugs Journal 2002;11(5):277‐9.
Wang 2003 {published data only}
-
- Wang XH, Zhang GW, Sen BS, Sun QF, Qiao HC. Bicyclol in the treatment of 110 patients with chronic hepatitis B. Infectious Disease Information 2003;16(4):188‐9.
Wu 2003 {published data only}
-
- Wu WF, Sun WW, Li DK. Effect of comparing bicyclol with bifendate in the treatment of chronic hepatitis B. Infectious Diseases Information 2003;16(4):186‐7.
Yao 2005 {published data only}
-
- Yao GB, Xu DZ, Lan P, Xu CB, Wang C, Luo J, et al. Efficacy and safety of bicyclol in treatment of 2200 patients with chronic viral hepatitis. Chinese Journal of New Drugs and Clinical Remedies 2005;24(6):421‐6.
Yu 2003 {published data only}
-
- Yu ZJ, Jiang HQ, Kan QC, Li XF. Dynamically analyzing on the anti‐virus and hepatoprotective effects of bicyclol. Chinese Pharmacoindustry 2003;12(8):27‐8.
Zhang 2005 {published data only}
-
- Zhang HF, Yang XJ, Zhu SS, Xu ZQ, Dong Y, Chen DW, et al. Study on treatment effectiveness and safety in children with chronic hepatitis B or C using bicyclol tablets. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2005;19(4):380‐2. - PubMed
Zhu 2002 {published data only}
-
- Zhu Y, Jin HY. Clinical research of bicyclol for treatment of chronic hepatitis B. Chinese New Drugs Journal 2002;11(6):486‐8.
Zhu 2003 {published data only}
-
- Zhu XH. Effect of bicyclol in the treatment of lamivudine dependence chronic hepatitis B. Infectious Diseases Information 2003;16(2):72.
Additional references
Alan 2005
-
- Franciscus A. Liver biopsy: grading and staging. http://www.hcvadvocate.org/hepatitis/Basics/Grade_Stage.pdf. Accessed in 20 Jan, 2005.
CFR & ICH 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice. 1997 CFR & ICH Guidelines. 1. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Chan 2005
-
- Chan HL, Leung NW, Hui AY, Wong VW, Liew CT, Chim AM, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon‐alpha2b and lamivudine with lamivudine alone. Annals of Internal Medicine 2005;142(4):240‐50. [MEDLINE: ] - PubMed
Cui 2002
-
- Cui S, Wang M, Fan G. Anti‐HBV efficacy of bifendate in treatment of chronic hepatitis B, a primary study. Zhonghua Yi Xue Za Zhi 2002;82(8):538‐40. - PubMed
Dusheiko 1999
-
- Dusheiko G. Hepatitis B. In: Bircher J, Benhamou JP, McIntyre N, Rizzetto M, Rodes J editor(s). Oxford Textbook of Clinical Hepatology. Vol. 1, Oxford: Oxford University Press, 1999:891.
Egger 1997
Gitlin 1997
-
- Gitlin N. Hepatitis B: diagnosis, prevention, and treatment. Clinical Chemistry 1997;43(8 Pt 2):1500‐6. - PubMed
Howard 2002
-
- Howard JW. Hepatitis B. http://cpmcnet.columbia.edu/dept/gi/hepB.html (accessed 11 June 2004).
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Janssen 2005
-
- Janssen HL, Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa‐2b alone or in combination with lamivudine for HBeAg‐positive chronic hepatitis B: a randomised trial. The Lancet 2005;365(9454):123‐9. [MEDLINE: ] - PubMed
Jüni 2001
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Liu 1999
-
- Liu GT, Zhang CZ, Li Y, Wei H, Hu W. Clinical and pharmacological study of bicyclol ‐ a potential anti‐choronic viral hepatitis drug. Chinese Medical Sciences Journal 1999;14(Suppl):51‐3.
Liu 2001
-
- Liu GT. The anti‐virus and hepatoprotective effect of bicyclol and its mechanism of action. Chinese Journal of New Drugs 2001;10(5):325‐7.
Liu 2002
-
- Liu J, Kjaergard LL, Gluud C. Misuse of randomization: a review of Chinese randomized trials of herbal medicines for chronic hepatitis B. American Journal of Chinese Medicine 2002;30(1):173‐6. - PubMed
Lok 2001
-
- Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology 2001;34(6):1225‐41. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. Lancet 1998;352:609‐13. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285(15):1987‐91. - PubMed
NNPP 2005
-
- The National New Products Program. National novel drug for anti hepatitis bicyclol. http://www.chinanp.gov.cn/cpzs/cpzs2‐10.htm accessed at 24 June, 2005.
Purcell 1993
-
- Purcell RH. The discovery of the hepatitis virus. Gastroenterology 1993;104(4):955‐63. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
WHO 2004
-
- WHO. Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en/ Accessed at 25 June, 2004.
Zhao 2001
-
- Zhao DM, Liu GT. Protective effect of bicyclol on concanavalin A induced liver nuclei DNA injury in mice. National Medical Journal of China 2001;81(14):844‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

