Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer
- PMID: 17054269
- PMCID: PMC8996243
- DOI: 10.1002/14651858.CD006019.pub2
Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer
Abstract
Background: Hormone therapy for early prostate cancer has demonstrated an improvement in clinical and pathological variables, but not always an improvement in overall survival. We performed a systematic review of both adjuvant and neo-adjuvant hormone therapy combined with surgery or radiotherapy in localised or locally advanced prostate cancer.
Objectives: The objective of this review was to undertake a systematic review and, if possible, a meta-analysis of neo-adjuvant and adjuvant hormone therapy in localised or locally advanced prostate cancer.
Search strategy: We searched MEDLINE (1966-2006), EMBASE, The Cochrane Library, Science Citation Index, LILACS, and SIGLE for relevant randomised trials. Handsearching of appropriate publications was also undertaken.
Selection criteria: Randomised or quasi-randomised controlled trials of patients with localised or locally advanced prostate cancer, that is, stages T1-T4, any N, M0, comparing neo-adjuvant or adjuvant hormonal deprivation in combination with primary therapy (radical radiotherapy or radical prostatectomy) versus primary therapy alone were included in this review.
Data collection and analysis: Data were extracted from eligible studies and assessed for quality, and included information on study design, participants, interventions, and outcomes. Comparable data were pooled together for meta-analysis with intention-to treat principle.
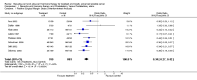
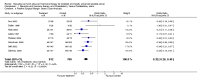
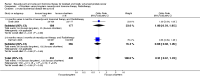
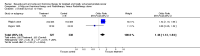
Main results: Men with prostate cancer have different clinical outcomes based on their risk (T1-T2, T3-T4, PSA levels and Gleason score). However, the majority of studies included in this review did not report results by risk groups; therefore, it was not possible to perform sub-group analysis. Neo-adjuvant hormonal therapy prior to prostatectomy did not improve overall survival (OR 1.11, 95% CI 0.67 to 1.85, P = 0.69). However, there was a significant reduction in the positive surgical margin rate (OR 0.34, 95% CI 0.27 to 0.42, P < 0.00001) and a significant improvement in other pathological variables such as lymph node involvement, pathological staging and organ confined rates. There was a borderline significant reduction of disease recurrence rates (OR 0.74, 95% CI 0.55 to 1.0, P = 0.05), in favour of treatment. The use of longer duration of neo-adjuvant hormones, that is either 6 or 8 months prior to prostatectomy, was associated with a significant reduction in positive surgical margins (OR 0.56, 95% CI 0.39 to 0.80, P = 0.002). In one study, neo-adjuvant hormones prior to radiotherapy significantly improved overall survival for Gleason 2 to 6 patients; although, in two studies, there was no improvement in disease-specific survival (OR 0.99, 95% CI 0.75 to 1.32, P = 0.97). However, there was a significant improvement in both clinical disease-free survival (OR 1.86, 95% CI 1.93 to 2.40, P < 0.00001) and biochemical disease-free survival (OR 1.93, 95% CI 1.45 to 2.56, P < 0.00001). Adjuvant androgen deprivation following prostatectomy did not significantly improve overall survival at 5 years (OR 1.50, 95% CI 0.79 to 2.85, P = 0.2); although one study reported a significant disease-specific survival advantage with adjuvant therapy (P = 0.001). In addition, there was a significant improvement in disease-free survival at both 5 years (OR 3.73, 95%CI 2.30 to 6.03, P < 0.00001) and 10 years (OR 2.06, 95% CI 1.34 to 3.15, P = 0.0009). Adjuvant therapy following radiotherapy resulted in a significant overall survival gain apparent at 5 (OR 1.46, 95% CI 1.17 to 1.83, P = 0.0009) and 10 years (OR 1.44, 95% CI 1.13 to 1.84, P = 0.003); although there was significant heterogeneity (P = 0.09 and P = 0.07, respectively). There was also a significant improvement in disease-specific survival (OR 2.10, 95% CI 1.53 to 2.88, P = 0.00001) and disease-free survival (OR 2.53, 95% CI 2.05 to 3.12, P < 0.00001) at 5 years.
Authors' conclusions: Hormone therapy combined with either prostatectomy or radiotherapy is associated with significant clinical benefits in patients with local or locally advanced prostate cancer. Significant local control may be achieved when given prior to prostatectomy or radiotherapy, which may improve patient's quality of life. When given adjuvant to these primary therapies, hormone therapy, not only provides a method for local control, but there is also evidence for a significant survival advantage. However, hormone therapy is associated with significant side effects, such as hot flushes and gynaecomastia, as well as cost implications. The decision to use hormone therapy should, therefore, be taken at a local level, between the patient, clinician and policy maker, taking into account the clinical benefits, toxicity and cost. More research is needed to guide the choice, the duration, and the schedule of hormonal deprivation therapy, and the impact of long-term hormone therapy with regard to toxicity and the patient's quality of life.
Conflict of interest statement
None.
Figures























Update of
References
References to studies included in this review
Aus 2002 {published data only}
-
- Hugosson J, Abrahamsson PA, Ahlgren G, Aus G, Lundberg S, Schelin S, Schain M, Pedersen K. The risk of malignancy in the surgical margin at radical prostatectomy reduced almost three‐fold in patients given neo‐adjuvant hormone treatment. European Urology 1996;29(4):413‐9. - PubMed
Bolla 2002 {published data only}
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Mattelaer J, Lopez Torecilla J, Pfeffer JR, Lino Cutajar C, Zurlo A, Pierart M. Long‐term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360(9327):103‐6. - PubMed
Crook 2004 {published data only}
-
- Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L, Bowen J, Robertson S, Lockwood G. Report of a multicenter Canadian phase III randomized trial of 3 months vs. 8 months neoadjuvant androgen deprivation before standard‐dose radiotherapy for clinically localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2004;60(1):15‐23. - PubMed
Dalkin 1996 {published data only}
-
- Dalkin BL, Ahmann FR, Nagle R, Johnson CS. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. Journal of Urology 1996;155(4):1357‐60. - PubMed
Denham 2005 {published data only}
-
- Denham JW, Steigler A, Lamb DS, Mameghan H, Turner S, Matthews J, Franklin I, Atkinson C, North J, Paulsen M, Christie D, Spry NA, Tai K‐H, Wynne C, Duchesne G, Kovacev O, D'Este C. Short‐term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans‐Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncology 2005;6:841‐50. - PubMed
Gleave 2001 {published data only}
-
- Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, Jewett M, Kassabian V, Chetner M, Dupont C, Rensselaer S. Randomized comparative study of 3 versus 8‐month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. Journal of Urology 2001;166(2):500‐6. - PubMed
Klotz 2003 {published data only}
-
- Klotz LH, Goldenberg SL, Jewett MAS, Fradet Y, Nam R, Barkin J, Chin J, Chatterjee S. Long‐term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. Journal of Urology 2003;170(3):791‐4. - PubMed
Labrie 1997 {published data only}
-
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay M, Tetu B, Fradet Y, Belanger A, Candas B. Neoadjuvant hormonal therapy: The Canadian experience. Urology 1997;49(3 Suppl):56‐64. - PubMed
Lamb 2003 {published data only}
-
- Lamb DS, Denham JW, Mameghan H, Joseph D, Turner S, Matthews J, Franklin I, Atkinson C, North J, Poulsen M, Kovacev O, Robertson R, Francis L, Christie D, Spry NA, Tai KH, Wynne C, Duchesne G. Acceptability of short term neo‐adjuvant androgen deprivation in patients with locally advanced prostate cancer. Radiotherapy and Oncology 2003;68(3):255‐67. - PubMed
Laverdiere 2004 {published data only}
-
- Laverdiere J, Nabid A, Bedoya LD, Ebacher A, Fortin A, Wang CS, Harel F. The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2‐T3 prostate cancer. Journal of Urology 2004;171(3):1137‐40. - PubMed
McLeod 2005 {published data only}
-
- McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP. Bicalutamide 150mg plus standard care vs standard care alone for early prostate cancer. British Journal Urology International 2005;97:247‐54. - PubMed
Messing 1999 {published data only}
-
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node‐positive prostate cancer.[see comment]. New England Journal of Medicine 1999;341(24):1781‐8. - PubMed
Pilepich 2001 {published data only}
-
- Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, Lawton C, Machtay M, Grignon D. Phase III radiation therapy oncology group (RTOG) trial 86‐10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2001;50(5):1243‐52. [MEDLINE: ] - PubMed
Pilepich 2005 {published data only}
-
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma ‐ Long‐term results of phase III RTOG 85‐31. International Journal of Radiation Oncology, Biology, Physics 2005;61(5):1285‐90. [0360‐3016] - PubMed
Prezioso 2004 {published data only}
-
- Prezioso D, Lotti T, Polito M, Montironi R. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: A randomized study. Urologia Internationalis 2004;72(3):189‐95. - PubMed
Schulman 2000 {published data only}
-
- Schulman CC, Debruyne FMJ, Forster G, Selvaggi FP, Zlotta AR, Witjes WPJ. 4‐Year follow‐up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2‐3N0M0 prostate cancer. European Urology 2000;38(6):706‐13. - PubMed
Selli 2002 {published data only}
-
- Selli C, Montironi R, Bono A, Pagano F, Zattoni F, Manganelli A, Selvaggi FP, Comeri G, Fiaccavento G, Guazzieri S, Lembo A, Cosciani‐Cunico S, Potenzoni D, Muto G, Mazzucchelli R, Santinelli A. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. Journal of Clinical Pathology 2002;55(7):508‐13. - PMC - PubMed
Soloway 2002 {published data only}
-
- Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, Puras‐Baez A, Lupron Depot Neoadjuvant Prostate Cancer Study G. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5‐year results. Journal of Urology 2002;167(1):112‐6. - PubMed
Tyrrell 2005 {published data only}
-
- Tyrrel CJ, Payne H, See W, McLeod DG, Wirth M, Iversen P, Armstrong J, Morris C, on behalf of the 'Casodex' early Prostate Trialist Group. Bicalutamide ('Casodex') 150mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: Results from the randomised Early Prostate Cancer Programme. Radiotherapy and Oncology 2005;76:4‐10. - PubMed
Van Der Kwast 1999 {published data only}
-
- Kwast TH, Tetu B, Candas B, Gomez JL, Cusan L, Labrie F. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: Three versus six months of endocrine therapy. Urology 1999;53(3):523‐9. - PubMed
Wirth 2004a {published data only}
-
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, Noack B, Hinke A, Froehner M. Prospective Randomized Trial Comparing Flutamide as Adjuvant Treatment versus Observation after Radical Prostatectomy for Locally Advanced, Lymph Node‐Negative Prostate Cancer. European Urology 2004;45(3):267‐70. - PubMed
Zagars 1988 {published data only}
-
- Zagars GK, Johnson DE, Eschenbach AC, Hussey DH. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: Long‐term results of a prospective randomized study. International Journal of Radiation Oncology, Biology, Physics 1988;14(6):1085‐91. - PubMed
References to studies excluded from this review
Ahlgren 1999 {published data only}
-
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson PA. Tumor cell proliferation in prostate cancer after 3 months of neoadjuvant LHRH analogue treatment is a prognostic marker of recurrence after radical prostatectomy. Urology 1999;54(2):329‐34. - PubMed
Ahlgren 2000a {published data only}
-
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson P. Neuroendocrine differentiation is not prognostic of failure after radical prostatectomy but correlates with tumor volume. Urology 2000;56(6):1011‐5. - PubMed
Ahlgren 2000b {published data only}
-
- Ahlgren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson PA. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 2000;42(4):274‐9. - PubMed
Akakura 1999 {published data only}
-
- Akakura K, Isaka S, Akimoto S, Ito H, Okada K, Hachiya T, Yoshida O, Arai Y, Usami M, Kotake T, Tobisu K, Ohashi Y, Sumiyoshi Y, Kakizoe T, Shimazaki J. Long‐term results of a randomized trial for the treatment of Stages B2 and C prostate cancer: radical prostatectomy versus external beam radiation therapy with a common endocrine therapy in both modalities. Urology 1999;54(2):313‐8. - PubMed
Akaza 2003 {published data only}
-
- Akaza H, Homma Y, Okada K, Yokoyama M, Usami M, Hirao Y, Tsushima T, Ohashi Y, Aso Y. A prospective and randomized study of primary hormonal therapy for patients with localized or locally advanced prostate cancer unsuitable for radical prostatectomy: results of the 5‐year follow‐up. BJU International 2003;91(1):33‐6. [1464‐4096] - PubMed
Akaza 2004 {published data only}
-
- Akaza H, Yamaguchi A, Matsuda T, Igawa M, Kumon H, Soeda A, Arai Y, Usami M, Naito S, Kanetake H, Ohashi Y. Superior anti‐tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone‐releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first‐line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Japanese Journal of Clinical Oncology 2004;34(1):20‐8. - PubMed
Anderson 1997 {published data only}
-
- Anderson PR, Hanlon AL, Movsas B, Hanks GE. Prostate cancer patient subsets showing improved bNED control with adjuvant androgen deprivation. International Journal of Radiation Oncology, Biology, Physics 1997;39(5):1025‐30. - PubMed
Andriole 1998 {published data only}
-
- Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, Hudson P, Jackson CL, Romas NA, Patterson L, Cook TJ, Waldstreicher J. Treatment with finasteride preserves usefulness of prostate‐specific antigen in the detection of prostate cancer: results of a randomized, double‐blind, placebo‐controlled clinical trial. PLESS Study Group. Proscar Long‐term Efficacy and Safety Study. Urology 1998;52(2):195‐201. - PubMed
Armstrong 2004 {published data only}
-
- Armstrong J, Taylor J, Thirion P, Henry P, Hollywood D, O' Neill L, Cosgrove S, Horan C, Stevenson M, Butler M. Preliminary results of a randomised trial comparing short vs. protracted neoadjuvant hormonal therapy (NHT) prior to radiation therapy (RT) of localized prostate cancer. (Irish Clinical Oncology Research Group Protocol # 97‐01). Radiotherapy and Oncology 2004;73:S78.
Asbell 1996 {published data only}
-
- Asbell SO, Leon SA, Tester WJ, Brereton HD, Ago CT, Rotman M. Development of anemia and recovery in prostate cancer patients treated with combined androgen blockade and radiotherapy. Prostate 1996;29(4):243‐8. - PubMed
Aus 1994 {published data only}
-
- Aus G, Brandstedt S, Haggman M, Pedersen K, Torre M, Hugosson J. Effects of 3 months' neoadjuvant hormonal treatment with a GnRH analogue (triptorelin) prior to radical retropubic prostatectomy on prostate‐specific antigen and tumour volume in prostate cancer. European Urology 1994;26(1):22‐8. [0302‐2838] - PubMed
Bolla 1997 {published data only}
-
- Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. New England Journal of Medicine 1997;337(5):295‐300. - PubMed
Bolla 1999 {published data only}
-
- Bolla M. Adjuvant hormonal treatment with radiotherapy for locally advanced prostate cancer. European Urology 1999;35 Suppl 1:23‐5; discussion 26. [MEDLINE: ] - PubMed
Bonney 1998 {published data only}
-
- Bonney WW, Schned AR, Timberlake DS. Neoadjuvant androgen ablation for localized prostatic cancer: Pathology methods, surgical end points and meta‐analysis of randomized trials. Journal of Urology 1998;160(5):1754‐60. - PubMed
Bono 2001 {published data only}
-
- Bono AV, Pagano F, Montironi R, Zattoni F, Manganelli A, Selvaggi FP, Comeri G, Fiaccavento G, Guazzieri S, Selli C, Lembo A, Cosciani‐Cunico S, Potenzoni D, Muto G, Diamanti L, Santinelli A, Mazzucchelli R, Prayer‐Galletti T. Effect of complete androgen blockade on pathologic stage and resection margin status of prostate cancer: Progress pathology report of the Italian PROSIT study. Urology 2001;57(1):117‐21. - PubMed
Bullock 2002 {published data only}
-
- Bullock MJ, Srigley JR, Klotz LH, Larry Goldenberg S. Pathologic effects of neoadjuvant cyproterone acetate on nonneoplastic prostate, prostatic intraepithelial neoplasia, and adenocarcinoma: A detailed analysis of radical prostatectomy specimens from a randomized trial. American Journal of Surgical Pathology 2002;26(11):1400‐13. - PubMed
Chang 2000 {published data only}
-
- Chang SS, Reuter VE, Heston WDW, Hutchinson B, Grauer LS, Gaudin PB. Short term neoadjuvant androgen deprivation therapy does not affect prostate specific membrane antigen expression in prostate tissues. Cancer 2000;88(2):407‐15. - PubMed
Cookson 1997 {published data only}
-
- Cookson MS, Sogani PC, Russo P, Sheinfeld J, Herr H, Dalbagni G, Reuter VE, Begg CB, Fair WR. Pathological staging and biochemical recurrence after neoadjuvant androgen deprivation therapy in combination with radical prostatectomy in clinically localized prostate cancer: results of a phase II study. British Journal of Urology 1997;79(3):432‐8. [0007‐1331] - PubMed
D'Amico 2004 {published data only}
-
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6‐Month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. Journal of the American Medical Association 2004;292(7):821‐7. - PubMed
De Leval 2002 {published data only}
-
- Leval J, Boca P, Yousef E, Nicolas H, Jeukenne M, Seidel L, Bouffioux C, Coppens L, Bonnet P, Andrianne R, Wlatregny D. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone‐naive prostate cancer: results of a prospective randomized multicenter trial. Clinical Prostate Cancer 2002;1(3):163‐71. - PubMed
Debruyne 1994 {published data only}
-
- Debruyne FMJ, Witjes WPJ, Schulman CC, Cangh PJ, Oosterhof GON. A multicentue trial of combined neoadjuvant androgen blockade with Zoladex and flutamide prior to radical prostatectomyin prostate cancer. European Urology 1994;26(Suppl 1):4. - PubMed
Debruyne 2000 {published data only}
-
- Debruyne FMJ, Witjes WPJ, Zietman A, Bruchovsky N, Klotz L, Fair W. Neoadjuvant hormonal therapy prior to radical prostatectomy: The European experience. Molecular Urology 2000;4(3):251‐7. - PubMed
Fellows 1992 {published data only}
-
- Fellows GJ, Clark PB, Beynon LL, Boreham J, Keen C, Parkinson MC, Peto R, Webb JN. Treatment of advanced localised prostatic cancer by orchiectomy, radiotherapy, or combined treatment. A Medical Research Council Study. Urological Cancer Working Party‐‐Subgroup on Prostatic Cancer. British Journal of Urology 1992;70(3):304‐9. - PubMed
Galalae 2004 {published data only}
-
- Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, Eulau S, Gustafson G, Gribble M, Kovacs G. Long‐term outcome by risk factors using conformal high‐dose‐rate brachytherapy (HDR‐BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2004;58(4):1048‐55. [0360‐3016] - PubMed
Goldenberg 1996 {published data only}
-
- Goldenberg SL, Klotz LH, Srigley J, Jewett MA, Mador D, Fradet Y, Barkin J, Chin J, Paquin JM, Bullock MJ, Laplante S. Randomized, prospective, controlled study comparing radical prostatectomy alone and neoadjuvant androgen withdrawal in the treatment of localized prostate cancer. Canadian Urologic Oncology Group. [see comment]. Journal of Urology 1996;156(3):873‐7. - PubMed
Granfors 1998 {published data only}
-
- Granfors T, Modig H, Damber JE, Tomic R. Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: a prospective randomized study. Journal of Urology 1998;159(6):2030‐4. - PubMed
Hanks 2003 {published data only}
-
- Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM, Shipley WU. Phase III trial of long‐term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92‐02. Journal of Clinical Oncology 2003;21(21):3972‐8. - PubMed
Homma 1999 {published data only}
-
- Homma Y, Akaza H, Okada K, Yokoyama M, Moriyama N, Usami M, Hirao Y, Tsushima T, Sakamoto A, Ohashi Y, Aso Y. Early results of radical prostatectomy and adjuvant endocrine therapy for prostate cancer with or without preoperative androgen deprivation. The Prostate Cancer Study Group. International Journal of Urology 1999;6(5):229‐37; discussion 238‐9. - PubMed
Homma 2004 {published data only}
-
- Homma Y, Akaza H, Okada K, Yokoyama M, Usami M, Hirao Y, Tsushima T, Sakamoto A, Ohashi Y, Aso Y, Prostate Cancer Study G. Endocrine therapy with or without radical prostatectomy for T1b‐T3N0M0 prostate cancer. International Journal of Urology 2004;11(4):218‐24. [0919‐8172] - PubMed
Horwitz 2001 {published data only}
-
- Horwitz EM, Winter K, Hanks GE, Lawton CA, Russell AH, Machtay M. Subset analysis of RTOG 85‐31 and 86‐10 indicates an advantage for long‐term vs. short‐term adjuvant hormones for patients with locally advanced nonmetastatic prostate cancer treated with radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2001;49(4):947‐56. - PubMed
Hugosson 1996 {published data only}
-
- Hugosson J, Abrahamsson PA, Ahlgren G, Aus G, Lundberg S, Schelin S, Schain M, Pedersen K. The risk of malignancy in the surgical margin at radical prostatectomy reduced almost three‐fold in patients given neo‐adjuvant hormone treatment. European Urology 1996;29:413‐9. - PubMed
Isaka 1994 {published data only}
-
- Isaka S, Shimazaki J, Akimoto S, Okada K, Yoshida O, Arai Y, Usami M, Kotake T, Tobisu K, Kakizoe T, et al. A prospective randomized trial for treating stages B2 and C prostate cancer: radical surgery or irradiation with neoadjuvant endocrine therapy. Japanese Journal of Clinical Oncology 1994;24(4):218‐23. - PubMed
Iversen 2002 {published data only}
-
- Iversen P, Tammela TLJ, Vaage S, Lukkarinen O, Lodding P, Bull‐Njaa T, Viitanen J, Hoisaeter P, Lundmo P, Rasmussen F, Johansson JE, Persson BE, Carroll K. A randomised comparison of bicalutamide ('Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non‐metastatic prostate cancer first report from the Scandinavian Prostatic Cancer Group Study no. 6. European Urology 2002;42(3):204‐11. - PubMed
Iversen 2004a {published data only}
-
- Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, Tammela TLJ, Tasdemir I, Morris T, Carroll K. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3‐Year median followup from the Scandinavian Prostate Cancer Group Study Number 6. Journal of Urology 2004;172(5 pt 1):1871‐6. - PubMed
Iversen 2004b {published data only}
-
- Iversen P, Wirth MP, See WA, McLeod DG, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DM, Delaere KP, Lundmo P, Tammela TL, Johansson JE, Morris T, Carroll K. Is the efficacy of hormonal therapy affected by lymph node status? data from the bicalutamide (Casodex) Early Prostate Cancer program. Urology 2004;63(5):928‐33. [1527‐9995] - PubMed
Jani 2003 {published data only}
-
- Jani AB, Kao J, Hellman S. Hormone therapy adjuvant to external beam radiotherapy for locally advanced prostate carcinoma: a complication‐adjusted number‐needed‐to‐treat analysis. Cancer 2003;98(11):2351‐61. - PubMed
Klotz 1999 {published data only}
-
- Klotz LH, Goldenberg SL, Jewett M, et al. CUOG randomised trial of neo‐adjuvant androgen ablation before radical prostatectomy: 36 month post treatment PSA results. Urology 1999;53:757. - PubMed
Klotz 2000 {published data only}
-
- Klotz L, Gleave M, Goldenberg SL, Garnick M, Moul J, Sylvester J, Stone N. Neoadjuvant hormone therapy: The Canadian trials. Molecular Urology 2000;4(3):233‐7. - PubMed
Labrie 1993a {published data only}
-
- Labrie F, Dupont A, Cusan L, Gomez J, Diamond P, Koutsilieris M, Suburu R, Fradet Y, Lemay M, Tetu B, Emond J, Candas B. Downstaging of localized prostate cancer by neoadjuvant therapy with flutamide and lupron: The first controlled and randomized trial. Clinical and Investigative Medicine. Medecine Clinique et Experimentale 1993;16(6):499‐509. - PubMed
Labrie 1993b {published data only}
-
- Labrie F, Belanger A, Simard J, Labrie C, Dupont A. Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first‐line therapy. Cancer 1993;71(3 Suppl):1059‐67. [0008‐543X] - PubMed
Labrie 1994 {published data only}
-
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay M, Tetu B, Fradet Y, Candas B. Down‐staging of early stage prostate cancer before radical prostatectomy: The first randomized trial of neoadjuvant combination therapy with flutamide and a luteinizing hormone‐releasing hormone agonist. Urology 1994;44(6 Suppl):29‐37.
Labrie 1995 {published data only}
-
- Labrie F, Cusan L, Gomez JL, Diamond P, Suburu R, Lemay MT, tu B, Fradet Y, Candas B. Downstaging by combination therapy with flutamide and an LHRH agonist before radical prostatectomy. Cancer Surveys 1995;23:149‐56. [MEDLINE: ] - PubMed
Lawton 1997 {published data only}
-
- Lawton CA, Winter K, Byhardt R, Sause WT, Hanks GE, Russell AH, Rotman M, Porter A, McGowan DG, DelRowe JD, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with D1 (pN+) adenocarcinoma of the prostate (results based on a national prospective randomized trial, RTOG 85‐31). International Journal of Radiation Oncology, Biology, Physics 1997;38(5):931‐9. - PubMed
Lawton 2001 {published data only}
-
- Lawton CA, Winter K, Murray K, Machtay M, Mesic JB, Hanks GE, Coughlin CT, Pilepich MV. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85‐31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. International Journal of Radiation, Oncology, Biology, Physics 2001;49(4):937‐46. - PubMed
Lawton 2005 {published data only}
-
- Lawton CA, Winter K, Grignon D, Pilepich MV. Androgen suppression plus radiation versus radiation alone for patients with stage D1/pathologic node‐positive adenocarcinoma of the prostate: updated results based on national prospective randomized trial Radiation Therapy Oncology Group 85‐31. Journal of Clinical Oncology 2005;23(4):800‐7. [0732‐183X] - PubMed
Lee 2002 {published data only}
-
- Lee LN, Stock RG, Stone NN. Role of hormonal therapy in the management of intermediate‐ to high‐risk prostate cancer treated with permanent radioactive seed implantation. International Journal of Radiation Oncology, Biology, Physics 2002;52(2):444‐52. - PubMed
Matveev 1993 {published data only}
-
- Matveev BP, Liazaliev KT. The combined treatment of patients with locally disseminated prostatic cancer. Urologiia i Nefrologiia 1993;4:21‐5. [MEDLINE: ] - PubMed
Mazzucchelli 2001 {published data only}
-
- Mazzucchelli R, Montironi R, Prezioso D, Bono AV, Ferrari P, Manganelli A, Morelli P, Vito ML, Santinelli A, Diamanti L, Lotti T, Polito M, Takeda Italian TAPSG. Surgical pathology examination of radical prostatectomy specimens. Updated protocol based on the Italian TAP study. Anticancer Research 2001;21(5):3599‐607. - PubMed
Montironi 1999 {published data only}
-
- Montironi R, Diamanti L, Santinelli A, Galetti‐Prayer T, Zattoni F, Selvaggi FP, Pagano F, Bono AV. Effect of total androgen ablation on pathologic stage and resection limit status of prostate cancer. Initial results of the Italian PROSIT study. Pathology, Research and Practice 1999;195(4):201‐8. - PubMed
Pilepich 1989 {published data only}
-
- Pilepich MV, Krall JM, John MJ, Rubin P, Porter AT, Marcial VA, Martz KL. Hormonal cytoreduction in locally advanced carcinoma of the prostate treated with definitive radiotherapy: Preliminary results of RTOG 83‐07. International Journal of Radiation Oncology, Biology, Physics 1989;16(3):813‐7. - PubMed
Pilepich 1993 {published data only}
-
- Pilepich MV. Phase III trial of hormone cytoreduction in conjunction with definative radiotherapy in loacally advanced prostate carcinoma: the emerging role of PSA in the assessment of outcome. abstract no: 193. International Journal of Radiation Oncology, Biology, Physics 1993;27(S1):246.
Pilepich 1995a {published data only}
-
- Pilepich MV, Buzydlowski JW, John MJ, Rubin P, McGowan DG, Marcial VA. Phase II trial of hormonal cytoreduction with megestrol and diethylstilbestrol in conjunction with radiotherapy for carcinoma of the prostate: Outcome results of RTOG 83‐07. International Journal of Radiation Oncology, Biology, Physics 1995;32(1):175‐80. - PubMed
Pilepich 1995b {published data only}
-
- Pilepich MV, Krall JM, al‐Sarraf M, John MJ, Doggett RL, Sause WT, Lawton CA, Abrams RA, Rotman M, Rubin P, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology Group. Urology 1995;45(4):616‐23. - PubMed
Pilepich 1997 {published data only}
-
- Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavourable prognosis carcinoma of the prostate treated with definitive radiotherapy: report of RTOG protocol 85 ‐31. Journal of Clinical Oncology 1997;15:1013‐21. - PubMed
Prezioso 1999 {published data only}
-
- Prezioso D, Lotti T, Montironi R, Polito M. Role of neoadjuvant treatment in clinically confined prostate cancer. Takeda NHT Italian Group. European Urology 1999;35(Suppl 1):17‐21; discussion 22. - PubMed
Rabbani 1998a {published data only}
-
- Rabbani F, Goldenberg SL, Klotz LH. Predictors of pathological stage before neoadjuvant androgen withdrawal therapy and radical prostatectomy. The Canadian Urologic Oncology Group. [see comment]. Journal of Urology 1998;159(3):925‐8. - PubMed
Rabbani 1998b {published data only}
-
- Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false‐negative sextant prostate biopsies. Journal of Urology 1998;159(4):1247‐50. - PubMed
Schmidt 1993 {published data only}
-
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Adjuvant therapy for localized prostate cancer. Cancer 1993;71(3 Suppl):1005‐13. [MEDLINE: ] - PubMed
Schmidt 1996a {published data only}
-
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Adjuvant therapy for clinical localized prostate cancer treated with surgery or irradiation. European Urology 1996;29(4):425‐33. - PubMed
Schmidt 1996b {published data only}
-
- Schmidt JD, Gibbons RP, Murphy GP, Bartolucci A. Evaluation of adjuvant estramustine phosphate, cyclophosphamide, and observation only for node‐positive patients following radical prostatectomy and definitive irradiation. Investigators of the National Prostate Cancer Project. Prostate 1996;28(1):51‐7. - PubMed
See 2002 {published data only}
-
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, Chodak G, Montie J, Tyrrell C, Wallace DMA, Delaere KPJ, Vaage S, Tammela TLJ, Lukkarinen O, Persson BE, Carroll K, Kolvenbag G. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: First analysis of the early prostate cancer program. Journal of Urology 2002;168(2):429‐35. - PubMed
See 2003 {published data only}
-
- See W, Iveresen P, Wirth M, McLeod D, Garside L, Morris T. Immediate treatment with bicalutamide 150mg as adjuvant therapy significantly reduces the risk of PSA progression in early prostate cancer. European Urology 2003;44:512‐8. - PubMed
Shipley 2002 {published data only}
-
- Shipley WU, Lu JD, Pilepich MV, Heydon K, Roach IM, Wolkov HB, Sause WT, Rubin P, Lawton CA, Machtay M. Effect of a short course of neoadjuvant hormonal therapy on the response to subsequent androgen suppression in prostate cancer patients with relapse after radiotherapy: A secondary analysis of the randomized protocol RTOG 86‐10. International Journal of Radiation Oncology, Biology, Physics 2002;54(5):1302‐10. - PubMed
Solomon 1993 {published data only}
-
- Solomon MH, McHugh TA, Dorr RP, Lee F, Siders DB. Hormone ablation therapy as neoadjuvant treatment to radical prostatectomy. Clinical and Investigative Medicine. Medecine Clinique et Experimentale 1993;16(6):532‐8. - PubMed
Soloway 1995 {published data only}
-
- Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood Jr DP, Puras‐Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. Journal of Urology 1995;154(2 I):424‐428. - PubMed
Vaillancourt 1996 {published data only}
-
- Vaillancourt L, Tetu B, Fradet Y, Dupont A, Gomez J, Cusan L, Suburu ER, Diamond P, Candas B, Labrie F. Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma: A randomized study. American Journal of Surgical Pathology 1996;20(1):86‐93. - PubMed
Van Poppel 1992 {published data only}
-
- Poppel H, Ameye F, Oyen R, Voorde W, Baert L. Neo‐adjuvant hormonotherapy does not facilitate radical prostatectomy. Acta Urologica Belgica 1992;60(3):73‐82. - PubMed
Van Poppel 1995 {published data only}
-
- Poppel H, Ridder D, Elgamal AA, Voorde W, Werbrouck P, Ackaert K, Oyen R, Pittomvils G, Baert L. Neoadjuvant hormonal therapy before radical prostatectomy decreases the number of positive surgical margins in stage T2 prostate cancer: interim results of a prospective randomized trial. The Belgian Uro‐Oncological Study Group. Journal of Urology 1995;154(2 Pt 1):429‐34. - PubMed
Van Poppel 2001 {published data only}
-
- Poppel H. Neoadjuvant hormone therapy and radical prostatectomy: The jury is still out. European Urology 2001;39(Suppl 1):10‐14. - PubMed
Wirth 2004b {published data only}
-
- Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K, Casodex Early Prostate Cancer Trialists G. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. Journal of Urology 2004;172(5 Pt 1):1865‐70. [0022‐5347] - PubMed
Witjes 1997 {published data only}
-
- Witjes WP, Schulman CC, Debruyne FM. Preliminary results of a prospective randomised study comparing radical prostatectomy versus radical prostatectomy associated with neo‐adjuvant hormonal combination therapy in T2‐3N0M0 prostatic carcinoma. Urology 1997;49(Suppl 3A):65. - PubMed
Yamanaka 2005 {published data only}
-
- Yamanaka H, Ito K, Naito S, Tsukamoto T, Usami M, Fujimoto H, Matsuoka N, Fukui I, Harada M, Ohashi Y, Kotake T, Kakizoe T. Effectiveness of adjuvant intermittent endocrine therapy following neoadjuvant endocrine therapy and external beam radiation therapy in men with locally advanced prostate cancer. Prostate 2005;63(1):56‐64. [0270‐4137] - PubMed
Zagars 1999 {published data only}
-
- Zagars GK, Pollack A, Smith LG. Conventional external‐beam radiation therapy alone or with androgen ablation for clinical stage III (T3, NX/N0, M0) adenocarcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 1999;44(4):809‐19. - PubMed
Zurlo 2002 {published data only}
-
- Zurlo A, Collette L, Tienhoven G, Blank LE, Warde P, Dubois JB, Jeanneret W, Storme G, Bernier J, Kuten A, Pierart M, Bolla M, Sternberg C, Kate FWJ, Mattelaer J, Lopez Torrecilla J, Pfeffer JR, Cutajar CL, Roberts T, Fossa S, Horiot JC, Hamers H, Bossett JF, Kovner F, Poppel H, Jager J, Svoboda V, Zambon JJ, Wijndaele JJ, Koriakine O, Boekenkruger C. Acute toxicity of conventional radiation therapy for high‐risk prostate cancer in EORTC trial 22863. European Urology 2002;42(2):125‐32. - PubMed
Additional references
Aus 1997
-
- Aus G, Hugosson J, Abrahamsson PA, et al. Pretreatment with triptorelin before radical prostatectomy: a 3 year follow up. Journal of Urology 1997;158(Suppl):393.
Baert 1998
-
- Baert LV, Goethuys HJ, Ridder DJ, et al. Neo‐adjuvant treatment before radical prostatectomy decreases the number of positive margins in CT2‐T3 but has no impact on PSA progression or survival in CT2‐T3. Journal of Urology 1998;159(Suppl):61.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Hanks 2000
-
- Hanks GE, Lu JD, Machtay, et al. RTOG protocol 9202: a phase 111 trial of the use of long term androgen suppression following neo adjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. International Journal of Radiation Oncology Biology and Physics 2000;48:112.
Huggins 1941
-
- Huggins C, Hodges CV. Studies on prostate cancer 1: the effect of castration, of oestrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research 1941;1:293‐7.
Jadad 1996
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [0197‐2456] - PubMed
Konski 2005
-
- Konski A, Sherman E, Krahn M, Bremner K, Beck JR, Watkins‐Bruner D, Pilepich M. Ecomomic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86‐10). International Journal Radiation Oncology Biology and Physics 2005;63(3):788‐94. - PubMed
Konski 2006
-
- Konski A, Watkins‐Bruner D, Brereton H, Feigenberg S, Hanks G. Long‐term hormone therapy and radiation is cost‐effective for patients with locally advanced prostate cancer. Cancer 2006;106:51‐7. - PubMed
Laverdiere 1997
-
- Laverdiere J, Gomez JL, Cusan L, et al. Beneficial effect of combination hormonal therapy administered prior and following external beam irradiation in localised prostate cancer. International Journal of Radiation Oncology Biology and Physics 1997;37:247‐52. - PubMed
Meng 2002
-
- Meng MV, Grosfeld GD, Carrol PR, Small EJ. Neoadjuvant Strategies for Prostate Cancer Prior Radical Prostatectomy. Seminars in Urological Oncology 2002;20(3, suppl 1):10‐18. - PubMed
Moher 1999
-
- Moher D, Cook DJ, Jadad AR, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta‐analyses.. Health Technology Assessment 1999;3(12):1‐98. - PubMed
Pilepich 2000
-
- Pilepich MV, Winter K, Byhardt RW, et al. Androgen ablation adjuvant to definitive radiotherapy in carcinoma of the prostate: year 2000 update of RTOG phase 111 studies 86‐10 and 85‐31. International Journal Radiation Oncology Biology Physics 2000;48(3 Suppl):169.
Samant 2003
-
- Samant RS, Dunscombe PB, Roberts GH. A cost‐outcome analysis of long‐term adjuvant gorserelin in addition to radiotherapy for locally advanced prostate cancer. Urological Oncology 2003;21(May ‐ June (3)):171‐7. - PubMed
Stone 1999
-
- Stone N, Stock RG. Prostate brachytherapy: treatment strategies. Journal of Urology 1999;162:421‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

