Vaccines for preventing malaria (pre-erythrocytic)
- PMID: 17054280
- PMCID: PMC6532586
- DOI: 10.1002/14651858.CD006198
Vaccines for preventing malaria (pre-erythrocytic)
Abstract
Background: Vaccines against all stages of the malaria parasite are in development, mainly for Plasmodium falciparum, which causes the most serious form of malaria. Pre-erythrocytic vaccines act to prevent or delay a malaria attack by attacking the sporozoite and liver stages before the parasite reaches the bloodstream.
Objectives: To assess the efficacy and safety of pre-erythrocytic malaria vaccines against any type of human malaria.
Search strategy: In March 2006, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2006, Issue 1), MEDLINE, EMBASE, LILACS, and the Science Citation Index. We also searched conference proceedings and reference lists of articles, and contacted organizations and researchers in the field.
Selection criteria: Randomized controlled trials comparing pre-erythrocytic vaccines with placebo, control vaccine, or routine antimalarial control measures in people of any age receiving an artificial challenge or natural exposure to malaria infection.
Data collection and analysis: Both authors independently assessed trial quality and extracted data. Results of meta-analyses were expressed as relative risks with 95% confidence intervals (CI) using an intention-to-treat analysis.
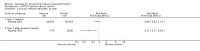
Main results: Nine safety and efficacy trials, and two safety trials, with over 3000 participants were included. In semi-immune children, RTS,S vaccine reduced clinical episodes of malaria by 26% (95% CI 13% to 37%) and severe malaria by 58% (95% CI 15% to 79%) for up to 18 months. Prevalence of parasitaemia was also reduced by 26% (95% CI 11% to 38%) at six months after immunization. RTS,S also reduced clinical malaria episodes by 63% (95% CI 18% to 83%) in semi-immune adult men in the second year of follow up after a booster dose. No severe adverse events were judged to be related to RTS,S vaccine, although the frequencies of injection site pain, swelling, arm motion limitation, headache, and malaise were increased in the vaccine groups. There was no evidence for effect of the CS-NANP vaccines (307 participants, 3 trials), CS102 peptide vaccine (14 participants, 1 trial), or the ME-TRAP vaccine (372 participants, 1 trial).
Authors' conclusions: RTS,S vaccine was effective in preventing a significant number of clinical malaria episodes, including good protection against severe malaria in children for 18 months. No severe adverse events were attributable to the vaccine. Progression of this vaccine towards licensing is justified while efforts to increase its efficacy continue. The other vaccines do not look promising and further research is a priority.
Conflict of interest statement
None known.
Figures
















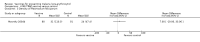


References
References to studies included in this review
Alonso 2005a {published data only}
-
- Alonso P, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 2004;364(9443):1411‐20. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, et al. Duration of protection with RTS,S ASO2A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single‐blind extended follow‐up of a randomised controlled trial. Lancet 2005;366(9502):2012‐8. - PubMed
Alonso 2005ab {published data only}
-
- See Alonso 2005a, 2005b.
Alonso 2005b {published data only}
-
- Alonso P, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and diseases in young African children: randomised controlled trial. Lancet 2004;364(9443):1411‐20. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, et al. Duration of protection with RTS,S ASO2A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single‐blind extended follow‐up of a randomised controlled trial. Lancet 2005;366(9502):2012‐8. - PubMed
Bojang 2001 {published data only}
-
- Alloueche A, Milligan P, Conway DJ, Pinder M, Bojang K, Doherty T, et al. Protective efficacy of the RTS,S/ASO2 Plasmodium falciparum malaria vaccine is not strain specific. American Journal of Tropical Medicine and Hygiene 2003;68(1):97‐101. - PubMed
-
- Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi‐immune adult men in The Gambia: a randomised trial. Lancet 2001;358(9297):1927‐34. - PubMed
Bojang 2005a {published data only}
-
- Bojang KA, Olodude F, Pinder M, Ofori‐Anyinam O, Vigneron L, Fitzpatrick S, et al. Safety and immunogenicity of RTS,S/ASO2A candidate malaria vaccine in Gambian children. Vaccine 2005;23(32):4148‐57. - PubMed
Bojang 2005b {published data only}
-
- Bojang KA, Olodude F, Pinder M, Ofori‐Anyinam O, Vigneron L, Fitspatrick S, et al. Safety and immunogenicity of RTS,S/ASO2A candidate malaria vaccine in Gambian children. Vaccine 2005;23(32):4148‐57. - PubMed
Brown 1994 {published data only}
-
- Brown AE, Singharaj P, Webster HK, Pipithkul J, Gordon DM, Boslego JW, et al. Safety, immunogenicity and limited efficacy study of a recombinant Plasmodium falciparum circumsporozoite vaccine in Thai soldiers. Vaccine 1994;12(2):102‐7. - PubMed
Genton 2005 {published and unpublished data}
-
- Genton B, D'Acremont V, Lurati F, Verhage D, Hermsen R, Audran R, et al. Randomized, double‐blind placebo‐controlled Phase IIa trial to test efficacy of the malaria vaccine PfCS102 to protect non‐immune volunteers against falciparum challenge. Acta Tropica 2005;Suppl 95:84.
Guiguemde 1990 {published data only}
-
- Guiguemde TR, Sturchler D, Ouedraogo JB, Drabo M, Etlinger H, Douchet C, et al. Vaccination against malaria: initial trial with an ant‐sporozoite vaccine, (NANP)3‐TT (RO 40‐2361) in Africa (Bobo‐Dioulasso, Burkina Faso) [Vaccination contre le paludisme: premier essai avec un vaccin antisporozoite, le (NANP)3‐TT (RO40‐2361) en Afrique (Bobo‐Dioulasso, Burkina Faso)]. Bulletin de la Societe de Pathologie Exotique 1990;83(2):217‐27. - PubMed
Kester 2001 {published data only}
-
- Kester KE, McKinney DA, Tornieporth N, Ockenhouse C, Heppner DG, Hall T, et al. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. Journal of Infectious Diseases 2001;183(4):640‐7. - PubMed
Moorthy 2004b {published data only}
Sherwood 1996 {published data only}
-
- Sherwood JA, Copeland RS, Taylor KA, Abok K, Oloo AJ, Were JB, et al. Plasmodium falciparum circumsporozoite vaccine immunogenicity and efficacy trial with natural challenge quantitation in an area of endemic human malaria in Kenya. Vaccine 1996;14(8):817‐27. - PubMed
References to studies excluded from this review
Bejon 2005 {published data only}
-
- Bejon P, Andrews L, Andersen RF, Dunachie S, Webster D, Walther M, et al. Calculation of liver‐to‐blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial polymerase chain reaction studies of volunteers challenged with malaria sporozoites. Journal of Infectious Diseases 2005;191(4):619‐26. - PubMed
Doherty 1999 {published data only}
-
- Doherty JF, Pinder M, Tornieporth N, Carton C, Vigneron L, Milligan P, et al. A phase I safety and immunogenicity trial with the candidate malaria vaccine RTS,S/SBAS2 in semi‐immune adults in the Gambia. American Journal of Tropical Medicine and Hygiene 1999;61(6):865‐8. - PubMed
Edelman 2002 {published data only}
-
- Edelman R, Wasserman SS, Kublin JG, Bodison SA, Nardin EH, Oliveira GA, et al. Immediate‐type hypersensitivity and other clinical reactions in volunteers immunized with a synthetic multi‐antigen peptide vaccine (PfCS‐MAP1NYU) against Plasmodium falciparum sporozoites. Vaccine 2002;21(3‐4):269‐80. - PubMed
Epstein 2004 {published data only}
-
- Epstein JE, Charoenvit Y, Kester K, Wang R, Newcomer R, Fitzpatrick S, et al. Safety, tolerability and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/ASO2A. Vaccine 2004;22(13‐4):1592‐603. - PubMed
Fries 1992 {published data only}
Gordon 1990 {published data only}
-
- Gordon DM, Cosgriff TM, Schneider I, Wasserman GF, Majarian WR, Hollingdale MR, et al. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. American Journal of Tropical Medicine and Hygiene 1990;42(6):527‐31. - PubMed
Gordon 1995 {published data only}
-
- Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, et al. Safety, immunogenicity and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein‐hepatitis B surface antigen subunit vaccine. Journal of Infectious Diseases 1995;171(6):1576‐85. - PubMed
Heppner 1996 {published data only}
-
- Heppner DG, Gordon DM, Gross M, Wellde B, Leitner W, Krzych U, et al. Safety, immunogenicity and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. Journal of Infectious Diseases 1996;174(2):361‐6. - PubMed
Hoffman 1994 {published data only}
-
- Hoffman SL, Edelman R, Bryan JP, Schneider I, Davis J, Sedegah M, et al. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalene as adjuvant. American Journal of Tropical Medicine and Hygiene 1994;51(5):603‐12. - PubMed
Hoffman 2002 {published data only}
-
- Hoffman SL, Goh ML, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation‐attenuated Plasmodium falciparum sporozoites. Journal of Infectious Diseases 2002;185(8):1155‐64. - PubMed
Le 1999 {published data only}
-
- Le TP, Coonan KM, Hedstrom RC, Charoenvit Y, Sedegah M, Epstein JE, et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 2000;18(18):1893‐901. - PubMed
McConkey 2003 {published data only}
-
- McConkey SJ, Reece WHH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T‐cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nature Medicine 2003;9(6):729‐35. - PubMed
Moorthy 2003 {published data only}
-
- Moorthy VS, Pinder M, Reece WH, Watkins K, Atabani S, et al. Safety and immunogenicity of DNA modified vaccinia virus Ankara malaria vaccination in African adults. Journal of Infectious Diseases 2003;188(8):1239‐44. - PubMed
Moorthy 2004c {published data only}
-
- Moorthy VS, Imoukhuede EB, Keating S, Pinder M, Webster D, Skinner MA, et al. Phase 1 evaluation of 3 highly immunogenic prime‐boost regimens, including a 12‐month reboosting vaccination, for malaria vaccination in Gambian men. Journal of Infectious Diseases 2004;189(12):2213‐9. - PubMed
Nardin 2000 {published data only}
-
- Nardin EH, Oliveira GA, Calvo‐Calle JM, Castro ZR, Nussenzweig RS, Schmeckpeper B, et al. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. Journal of Infectious Diseases 2000;182(5):1486‐96. - PubMed
Nardin 2001 {published data only}
-
- Nardin EH, Calvo‐Calle JM, Oliveira GA, Nussenzweig RS, Schneider M, Tiercy JM, et al. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. Journal of Immunology 2001;166(1):481‐9. - PubMed
Roggero 1999 {published data only}
-
- Roggero MA, Weilenmann C, Bonelo A, Audran R, Renggli J, Spertini F, et al. Plasmodium falciparium CS C‐terminal fragment: preclinical evaluation and phase I clinical studies. Parassitologia 1999;41(1‐3):421‐4. - PubMed
Stoute 1998 {published data only}
-
- Nussenzweig R, Zavala F. A malaria vaccine based on a sporozoite antigen. New England Journal of Medicine 1997;336(2):128‐30. - PubMed
-
- Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, et al. Long‐term efficacy and immune response following immunization with the RTS,S malaria vaccine. Journal of Infectious Diseases 1998;178(4):1139‐44. - PubMed
-
- Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S malaria vaccine evaluation group. New England Journal of Medicine 1997;336(2):86‐91. - PubMed
Walther 2005 {published data only}
-
- Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, et al. Safety, immunogenicity and efficacy of a pre‐erythrocytic malaria candidate vaccine, ICC‐1132 formulated in Seppic ISA 720. Vaccine 2005;23(7):857‐864. - PubMed
Additional references
Ballou 2004
-
- Ballou WR, Arrevalo‐Herrera M, Carucci D, Richie TL, Corradin G, Diggs C, et al. Update on the clinical development of candidate malaria vaccines. American Journal of Tropical Medicine and Hygiene 2004;71 Suppl 2:239‐47. - PubMed
Breman 2004
-
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. American Journal of Tropical Medicine and Hygiene 2004;71 Suppl 2:1‐15. - PubMed
Graves 2006a
Graves 2006b
Hay 2004
Higgins 2005
-
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 September 2005).
Jüni 2001
Moorthy 2004a
-
- Moorthy VS, Good MF, Hill AV. Malaria vaccine developments. Nature 2004;363(9403):150‐6. - PubMed
Review Manager 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Richie 2002
-
- Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature 2002;415(6872):694‐701. - PubMed
Snow 2004
-
- Snow RW, Korenromp E, Gouws E. Pediatric mortality in Africa: Plasmodium falciparum as a cause or risk?. American Journal of Tropical Medicine and Hygiene 2004;71 Suppl 2:16‐24. - PubMed
Snow 2005
WHO 2002
-
- World Health Organization. The world health report : 2002 : Reducing the risks, promoting healthy life. Geneva: World Health Organization, 2002.
WHO 2005
-
- World Health Organization. Initiative for Vaccine Research. Portfolio of candidate malaria vaccines currently in development: March 2005. www.who.int/vaccine_research/documents/en/malaria_table.pdf (accessed 3 May 2006).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

