Quantitation of rare memory B cell populations by two independent and complementary approaches
- PMID: 17055526
- PMCID: PMC1850941
- DOI: 10.1016/j.jim.2006.09.005
Quantitation of rare memory B cell populations by two independent and complementary approaches
Abstract
Current methodology for quantitation of memory B cells (MBC) in clinical samples is limited and often does not allow for detection of multiple MBC specificities in a single assay. Here we describe two independent approaches to antigen-specific MBC quantitation. First, a sensitive flow cytometry (FC) assay was developed for simultaneous quantitation of two prototypical B cell antigens, tetanus and diphtheria. Second, an ELISA-based MBC limiting dilution assay (LDA) was developed that provides quantitative analysis of up to 8-12 different MBC frequencies from a single blood sample. Cross-validation studies indicated that MBC numbers measured by FC correlated significantly with the frequencies obtained by LDA (R(2)=0.92, p=0.0002). These two functionally distinct approaches will be useful for accurate quantitation of rare MBC populations specific for either simple antigens (e.g. tetanus and diphtheria) or complex antigens (e.g. vaccinia or other viruses), and is amenable for use in a variety of model systems.
Figures


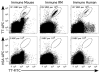

References
-
- Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunological reviews. 2006 In press. - PubMed
-
- Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–2. - PubMed
-
- Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. - PubMed
-
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

