Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia
- PMID: 17056551
- PMCID: PMC3912571
- DOI: 10.4049/jimmunol.177.9.6215
Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia
Abstract
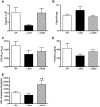
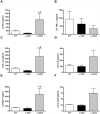
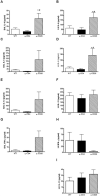
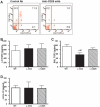
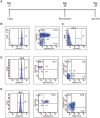
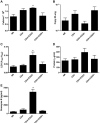
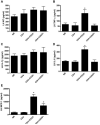
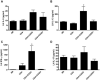
CD4+CD25+FoxP3+ regulatory T cells are decreased in patients infected with HIV and have been shown to be critical in mediating Ag tolerance in the lung. Because a subset of Pneumocystis-infected individuals develop substantial lung injury, which can be modeled in immune reconstituted scid mice, we used mouse models of Pneumocystis carinii to investigate the role of regulatory T cells in opportunistic infection and immune reconstitution. In this study, we show that CD4+CD25+FoxP3+ cells are part of the host response to Pneumocystis in CD4+ T cell-intact mice. Moreover, lung injury and proinflammatory Th1 and Th2 cytokine levels in the bronchoalveolar lavage fluid and lung homogenate were increased following CD4+CD25- immune reconstitution in Pneumocystis-infected SCID mice but not in CD4+CD25+ T cell-reconstituted animals. The ability of CD4+CD25+ T cells to control inflammation and injury during the course of Pneumocystis was confirmed by treatment of wild-type C57BL/6 mice with anti-CD25 mAb. These data show that CD4+CD25+ T cells control pulmonary inflammation and lung injury associated with Pneumocystis infection both in the setting of immune reconstitution as well as new acquisition of infection.
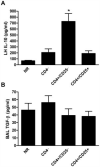
Figures

References
-
- Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1: Multicenter AIDS Cohort Study Group. N. Engl. J. Med. 1990;322:161–165. - PubMed
-
- Stansell JD, Osmond DH, Charlebois E, LaVange L, Wallace JM, Alexander BV, Glassroth J, Kvale PA, Rosen MJ, Reichman LB, et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons: pulmonary complications of HIV Infection Study Group. Am. J. Respir. Crit. Care Med. 1997;155:60–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

