VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation
- PMID: 17056569
- PMCID: PMC2711556
- DOI: 10.4049/jimmunol.177.9.6379
VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation
Abstract
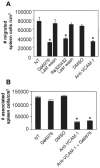
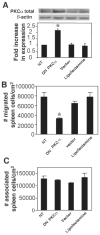
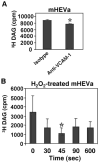
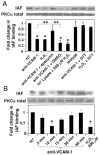
Lymphocyte binding to VCAM-1 activates endothelial cell NADPH oxidase, resulting in the generation of 1 muM H(2)O(2). This is required for VCAM-1-dependent lymphocyte migration. In this study, we identified a role for protein kinase Calpha (PKCalpha) in VCAM-1 signal transduction in human and mouse endothelial cells. VCAM-1-dependent spleen cell migration under 2 dynes/cm(2) laminar flow was blocked by pretreatment of endothelial cells with dominant-negative PKCalpha or the PKCalpha inhibitors, Rö-32-0432 or Gö-6976. Phosphorylation of PKCalpha(Thr638), an autophosphorylation site indicating enzyme activity, was increased by Ab cross-linking of VCAM-1 on endothelial cells or by the exogenous addition of 1 muM H(2)O(2). The anti-VCAM-1-stimulated phosphorylation of PKCalpha(Thr638) was blocked by scavenging of H(2)O(2) and by inhibition of NADPH oxidase. Furthermore, anti-VCAM-1 signaling induced the oxidation of endothelial cell PKCalpha. Oxidized PKCalpha is a transiently active form of PKCalpha that is diacylglycerol independent. This oxidation was blocked by inhibition of NADPH oxidase. In summary, VCAM-1 activation of endothelial cell NADPH oxidase induces transient PKCalpha activation that is necessary for VCAM-1-dependent transendothelial cell migration.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures

References
-
- Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and inter-cellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. - PubMed
-
- Mueller JP, Evans MJ, Cofiell R, Rother RP, Matis LA, Elliott EA. Porcine vascular cell adhesion molecule (VCAM) mediates endothelial cell adhesion to human T cells: development of blocking antibodies specific for porcine VCAM. Transplantation. 1995;60:1299–1306. - PubMed
-
- Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, et al. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. - PubMed
-
- Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

