Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia
- PMID: 17056580
- PMCID: PMC7611733
- DOI: 10.4049/jimmunol.177.9.6480
Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia
Abstract
Regulation of the inflammatory infiltrate is critical to the successful outcome of pneumonia. Alveolar macrophage apoptosis is a feature of pneumococcal infection and aids disease resolution. The host benefits of macrophage apoptosis during the innate response to bacterial infection are incompletely defined. Because NO is required for optimal macrophage apoptosis during pneumococcal infection, we have explored the role of macrophage apoptosis in regulating inflammatory responses during pneumococcal pneumonia, using inducible NO synthase (iNOS)-deficient mice. iNOS(-/-) mice demonstrated decreased numbers of apoptotic macrophages as compared with wild-type C57BL/6 mice following pneumococcal challenge, greater recruitment of neutrophils to the lung and enhanced expression of TNF-alpha. Pharmacologic inhibition of iNOS produced similar results. Greater pulmonary inflammation was associated with greater levels of early bacteremia, IL-6 production, lung inflammation, and mortality within the first 48 h in iNOS(-/-) mice. Labeled apoptotic alveolar macrophages were phagocytosed by resident macrophages in the lung and intratracheal instillation of exogenous apoptotic macrophages decreased neutrophil recruitment in iNOS(-/-) mice and decreased TNF-alpha mRNA in lungs and protein in bronchial alveolar lavage, as well as chemokines and cytokines including IL-6. These changes were associated with a lower probability of mice becoming bacteremic. This demonstrates the potential of apoptotic macrophages to down-regulate the inflammatory response and for the first time in vivo demonstrates that clearance of apoptotic macrophages decreases neutrophil recruitment and invasive bacterial disease during pneumonia.
Figures

 C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.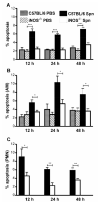
 C57BL/6. PBS,
C57BL/6. PBS,  iNOS-/- PBS,
iNOS-/- PBS,  C57BL/6 Spn,
C57BL/6 Spn,  iNOS-/- Spn.
iNOS-/- Spn.
 C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.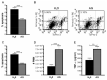
 H2O,
H2O,  AG.
AG.
 C57BL/6,
C57BL/6,  iNOS-/-.
iNOS-/-.

 C57BL/6 PBS,
C57BL/6 PBS,  iNOS-/- PBS,
iNOS-/- PBS,  C57BL/6 Spn,
C57BL/6 Spn,  iNOS-/- Spn.
iNOS-/- Spn.
 C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.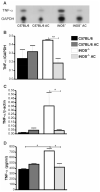
 C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.
 C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.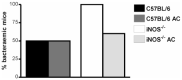
 C57BL/6,
C57BL/6,  C57BL/6 AC,
C57BL/6 AC,  iNOS-/-,
iNOS-/-,  iNOS-/- AC.
iNOS-/- AC.References
-
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. - PubMed
-
- Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

