Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes
- PMID: 17056587
- PMCID: PMC2174608
- DOI: 10.4049/jimmunol.177.9.6548
Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes
Abstract
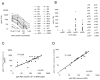
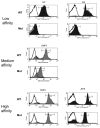
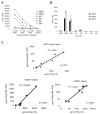
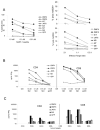
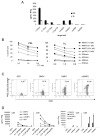
Cell-based antitumor immunity is driven by CD8(+) cytotoxic T cells bearing TCR that recognize specific tumor-associated peptides bound to class I MHC molecules. Of several cellular proteins involved in T cell:target-cell interaction, the TCR determines specificity of binding; however, the relative amount of its contribution to cellular avidity remains unknown. To study the relationship between TCR affinity and cellular avidity, with the intent of identifying optimal TCR for gene therapy, we derived 24 MART-1:27-35 (MART-1) melanoma Ag-reactive tumor-infiltrating lymphocyte (TIL) clones from the tumors of five patients. These MART-1-reactive clones displayed a wide variety of cellular avidities. alpha and beta TCR genes were isolated from these clones, and TCR RNA was electroporated into the same non-MART-1-reactive allogeneic donor PBMC and TIL. TCR recipient cells gained the ability to recognize both MART-1 peptide and MART-1-expressing tumors in vitro, with avidities that closely corresponded to the original TCR clones (p = 0.018-0.0003). Clone DMF5, from a TIL infusion that mediated tumor regression clinically, showed the highest avidity against MART-1 expressing tumors in vitro, both endogenously in the TIL clone, and after RNA electroporation into donor T cells. Thus, we demonstrated that the TCR appeared to be the core determinant of MART-1 Ag-specific cellular avidity in these activated T cells and that nonreactive PBMC or TIL could be made tumor-reactive with a specific and predetermined avidity. We propose that inducing expression of this highly avid TCR in patient PBMC has the potential to induce tumor regression, as an "off-the-shelf" reagent for allogeneic melanoma patient gene therapy.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures

References
-
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. - PubMed
-
- Boon T, Coulie PG, Eynde BJ, Bruggen PV. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. - PubMed
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. - PubMed
-
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

