Yeast mitochondrial ADP/ATP carriers are monomeric in detergents
- PMID: 17056710
- PMCID: PMC1618811
- DOI: 10.1073/pnas.0607640103
Yeast mitochondrial ADP/ATP carriers are monomeric in detergents
Abstract
Mitochondrial carriers are believed widely to be homodimers both in the inner membrane of the organelle and in detergents. The dimensions and molecular masses of the detergent and protein-detergent micelles were measured for yeast ADP/ATP carriers in a range of different detergents. The radius of the carrier at the midpoint of the membrane, its average radius, its Stokes' radius, its molecular mass, and its excluded volume were determined. These parameters are consistent with the known structural model of the bovine ADP/ATP carrier and they demonstrate that the yeast mitochondrial ADP/ATP carriers are monomeric in detergents. Therefore, models of substrate transport have to be considered in which the carrier operates as a monomer rather than as a dimer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
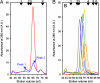


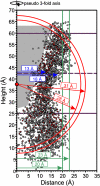
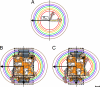
References
-
- Palmieri F. Pflugers Arch. 2004;447:689–709. - PubMed
-
- Kunji ER. FEBS Lett. 2004;564:239–244. - PubMed
-
- Saraste M, Walker JE. FEBS Lett. 1982;144:250–254. - PubMed
-
- Kunji ER, Harding M. J Biol Chem. 2003;278:36985–36988. - PubMed
-
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Nature. 2003;426:39–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

