Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors
- PMID: 17056720
- PMCID: PMC1618814
- DOI: 10.1073/pnas.0607761103
Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors
Abstract
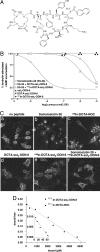
Targeting neuroendocrine tumors expressing somatostatin receptor subtypes (sst) with radiolabeled somatostatin agonists is an established diagnostic and therapeutic approach in oncology. While agonists readily internalize into tumor cells, permitting accumulation of radioactivity, radiolabeled antagonists do not, and they have not been considered for tumor targeting. The macrocyclic chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was coupled to two potent somatostatin receptor-selective peptide antagonists [NH(2)-CO-c(DCys-Phe-Tyr-DAgl(8)(Me,2-naphthoyl)-Lys-Thr-Phe-Cys)-OH (sst(3)-ODN-8) and a sst(2)-selective antagonist (sst(2)-ANT)], for labeling with (111/nat)In. (111/nat)In-DOTA-sst(3)-ODN-8 and (111/nat)In-DOTA-[4-NO(2)-Phe-c(DCys-Tyr-DTrp-Lys-Thr-Cys)-DTyr-NH(2)] ((111/nat)In-DOTA-sst(2)-ANT) showed high sst(3)- and sst(2)-binding affinity, respectively. They did not trigger sst(3) or sst(2) internalization but prevented agonist-stimulated internalization. (111)In-DOTA-sst(3)-ODN-8 and (111)In-DOTA-sst(2)-ANT were injected intravenously into mice bearing sst(3)- and sst(2)-expressing tumors, and their biodistribution was monitored. In the sst(3)-expressing tumors, strong accumulation of (111)In-DOTA-sst(3)-ODN-8 was observed, peaking at 1 h with 60% injected radioactivity per gram of tissue and remaining at a high level for >72 h. Excess of sst(3)-ODN-8 blocked uptake. As a control, the potent agonist (111)In-DOTA-[1-Nal(3)]-octreotide, with strong sst(3)-binding and internalization properties showed a much lower and shorter-lasting uptake in sst(3)-expressing tumors. Similarly, (111)In-DOTA-sst(2)-ANT was injected into mice bearing sst(2)-expressing tumors. Tumor uptake was considerably higher than with the highly potent sst(2)-selective agonist (111)In-diethylenetriaminepentaacetic acid-[Tyr(3),Thr(8)]-octreotide ((111)In-DTPA-TATE). Scatchard plots showed that antagonists labeled many more sites than agonists. Somatostatin antagonist radiotracers therefore are preferable over agonists for the in vivo targeting of sst(3)- or sst(2)-expressing tumors. Antagonist radioligands for other peptide receptors need to be evaluated in nuclear oncology as a result of this paradigm shift.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
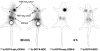

References
-
- Reubi JC. Endocr Rev. 2003;24:389–427. - PubMed
-
- Koenig JA, Edwardson JM. Trends Pharmacol Sci. 1997;18:276–287. - PubMed
-
- Cescato R, Schulz S, Waser B, Eltschinger V, Rivier J, Wester HJ, Culler M, Ginj M, Liu Q, Schonbrunn A, et al. J Nucl Med. 2006;47:502–511. - PubMed
-
- Bodei L, Paganelli G, Mariani G. J Nucl Med. 2006;47:375–377. - PubMed
-
- Hofland LJ, Lamberts SW. Endocr Rev. 2003;24:28–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

