Coordinate expression of the acetyl coenzyme A carboxylase genes, accB and accC, is necessary for normal regulation of biotin synthesis in Escherichia coli
- PMID: 17056747
- PMCID: PMC1797400
- DOI: 10.1128/JB.01373-06
Coordinate expression of the acetyl coenzyme A carboxylase genes, accB and accC, is necessary for normal regulation of biotin synthesis in Escherichia coli
Abstract
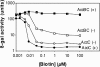
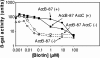
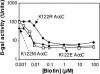
Transcription of the biotin (bio) biosynthetic operon of Escherichia coli is negatively regulated by the BirA protein, an atypical repressor protein in that it is also an enzyme. The BirA-catalyzed reaction involves the covalent attachment of biotin to AccB, a subunit of acetyl coenzyme (acetyl-CoA) carboxylase. The two functions of BirA allow regulation of the bio operon to respond to the intracellular concentrations of both biotin and unbiotinylated AccB. We report here that bio operon expression is down-regulated by overproduction of AccC, another acetyl-CoA carboxylase subunit known to form a complex with AccB. This down-regulation is eliminated when AccB and AccC are coordinately overexpressed, but only when the AccB partner is competent to bind AccC. Under AccC overexpression conditions AccB is underbiotinylated. These findings can be explained by a model in which excess AccC sequesters AccB in a complex that is a poor substrate for biotinylation. The observed disruption of biotin synthesis and attachment provides an excellent rationale for the observation that in the vast majority of sequenced bacterial genomes AccB and AccC are encoded in a two-gene operon.
Figures




References
-
- Barker, D. F., and A. M. Campbell. 1981. The birA gene of Escherichia coli encodes a biotin holoenzyme synthetase. J. Mol. Biol. 146:451-467. - PubMed
-
- Barker, D. F., and A. M. Campbell. 1981. Genetic and biochemical characterization of the birA gene and its product: evidence for a direct role of biotin holoenzyme synthetase in repression of the biotin operon in Escherichia coli. J. Mol. Biol. 146:469-492. - PubMed
-
- Beckett, D. 2005. The Escherichia coli biotin regulatory system: a transcriptional switch. J. Nutr. Biochem. 16:411-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

