Intravitreal clearance of moxifloxacin
- PMID: 17057790
- PMCID: PMC1447561
Intravitreal clearance of moxifloxacin
Abstract
Purpose: To study the clearance of a single dose of intravitreally injected moxifloxacin in rabbits.
Methods: Intravitreal injections of 200 microg/0.1 mL of moxifloxacin were performed in rabbits. Four eyes per time interval after injection (1, 6, 12, 24, 36 hours) and three eyes at 48 hours were enucleated, immediately frozen, and placed at -80 degrees C. Ocular dissection and isolation of frozen vitreous were performed. Antibiotic assays were performed with use of high-performance liquid chromatography.
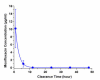
Results: The concentration of intravitreal moxifloxacin showed an exponential decay with a half-life of 1.72 hours. The mean vitreous concentration was 120.49 +/- 49.23 microg/mL 1 hour after injection, which declined to 20.23 +/- 5.85 microg/mL at 6 hours and 1.06 +/- 0.81 microg/mL at 12 hours. The aqueous levels of moxifloxacin showed an exponential decay from 10 microg/mL at 1 hour after intravitreal injection to undetectable levels by 12 hours after injection.
Conclusions: Moxifloxacin clearance from the vitreous is rapid and consistent with previous clearance studies of ciprofloxacin. Given that the injected dose corresponds to several times the minimum inhibitory concentration at which 90% of isolates are inhibited (MIC90) of organisms commonly involved in endophthalmitis, and that therapeutic levels are present up to 12 hours after injection, intravitreal moxifloxacin may have a role in the treatment of endophthalmitis.
Figures
References
-
- Endophthalmitis Vitrectomy Study Group. . Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravitreous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–1496. - PubMed
-
- Aaberg TM, Flynn HW, Jr, Murray TG. Intraocular ceftazidime as an alternative to the aminoglycosides in the treatment of endophthalmitis. Arch Ophthalmol. 1994;112:18–19. - PubMed
-
- Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–17. - PubMed
-
- Benz MS, Scott IU, Flynn HW, Jr, et al. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. - PubMed
-
- Kunimoto DY, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am J Ophthalmol. 1999;128:240–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources