Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia
- PMID: 17058262
- PMCID: PMC1948978
- DOI: 10.1002/hep.21366
Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia
Abstract
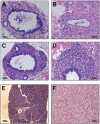
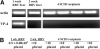
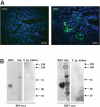
Biliary atresia is an inflammatory fibrosclerosing lesion of the bile ducts that leads to biliary cirrhosis and is the most frequent indication for liver transplantation in children. The pathogenesis of biliary atresia is not known; one theory is that of a virus-induced, subsequent autoimmune-mediated injury of bile ducts. The aim of this study was to determine whether autoreactive T cells and autoantibodies specific to bile duct epithelia are present in the rotavirus (RRV)- induced murine model of biliary atresia and whether the T cells are sufficient to result in bile duct inflammation. In vitro analyses showed significant increases in IFN-gamma-producing T cells from RRV-diseased mice in response to bile duct epithelial autoantigen. Adoptive transfer of the T cells from RRV-diseased mice into naïve syngeneic SCID recipients resulted in bile duct-specific inflammation. This induction of bile duct pathology occurred in the absence of detectable virus, indicating a definite response to bile duct autoantigens. Furthermore, periductal immunoglobulin deposits and serum antibodies reactive to bile duct epithelial protein were detected in RRV-diseased mice. In conclusion, both cellular and humoral components of autoimmunity exist in murine biliary atresia, and the progressive bile duct injury is due in part to a bile duct epithelia-specific T cell-mediated immune response. The role of cellular and humoral autoimmunity in human biliary atresia and possible interventional strategies therefore should be the focus of future research.
Figures



References
-
- Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, Mieli-Vergani G, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017–1025. - PubMed
-
- Tracy TF, Dillon P, Fox ES, Minnick K, Vogler C. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg. 1996;31:121–125. - PubMed
-
- Kobayashi H, Puri P, O'Briain DS, Surana R, Miyano T. Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg. 1997;32:590–593. - PubMed
-
- Karrer FM, Price MR, Bensard DD, Sokol RJ, Narkewicz MR, Smith DJ, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131:493–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
