Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase
- PMID: 17059388
- PMCID: PMC1798437
- DOI: 10.1042/BJ20060793
Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase
Abstract
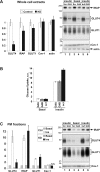
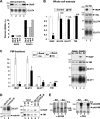
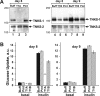
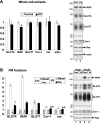
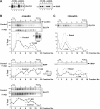
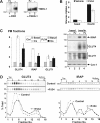
The glucose transporter GLUT4 and the aminopeptidase IRAP (insulin-responsive aminopeptidase) are the major cargo proteins of GSVs (GLUT4 storage vesicles) in adipocytes and myocytes. In the basal state, most GSVs are sequestered in perinuclear and other cytosolic compartments. Following insulin stimulation, GSVs undergo exocytic translocation to insert GLUT4 and IRAP into the plasma membrane. The mechanisms regulating GSV trafficking are not fully defined. In the present study, using 3T3-L1 adipocytes transfected with siRNAs (small interfering RNAs), we show that insulin-stimulated IRAP translocation remained intact despite substantial GLUT4 knockdown. By contrast, insulin-stimulated GLUT4 translocation was impaired upon IRAP knockdown, indicating that IRAP plays a role in GSV trafficking. We also show that knockdown of tankyrase, a Golgi-associated IRAP-binding protein that co-localizes with perinuclear GSVs, attenuated insulin-stimulated GSV translocation and glucose uptake without disrupting insulin-induced phosphorylation cascades. Moreover, iodixanol density gradient analyses revealed that tankyrase knockdown altered the basal-state partitioning of GLUT4 and IRAP within endosomal compartments, apparently by shifting both proteins toward less buoyant compartments. Importantly, the afore-mentioned effects of tankyrase knockdown were reproduced by treating adipocytes with PJ34, a general PARP (poly-ADP-ribose polymerase) inhibitor that abrogated tankyrase-mediated protein modification known as poly-ADP-ribosylation. Collectively, these findings suggest that physiological GSV trafficking depends in part on the presence of IRAP in these vesicles, and that this process is regulated by tankyrase and probably its PARP activity.
Figures
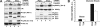
References
-
- Bryant N. J., Govers R., James D. E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 2002;3:267–277. - PubMed
-
- Keller S. R. The insulin-regulated aminopeptidase: a companion and regulator of GLUT4. Front. Biosci. 2003;8:S410–S420. - PubMed
-
- Ducluzeau P. H., Fletcher L. M., Vidal H., Laville M., Tavare J. M. Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab. 2002;28:85–92. - PubMed
-
- Mora S., Pessin J. E. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 2002;18:345–356. - PubMed
-
- Chen D., Elmendorf J. S., Olson A. L., Li X., Earp H. S., Pessin J. E. Osmotic shock stimulates GLUT4 translocation in 3T3L1 adipocytes by a novel tyrosine kinase pathway. J. Biol. Chem. 1997;272:27401–27410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

