The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity
- PMID: 17060455
- PMCID: PMC1800642
- DOI: 10.1128/MCB.00985-06
The Rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity
Abstract
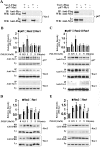
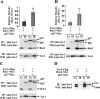
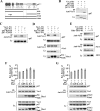
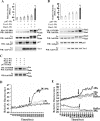
The phagocyte NADPH oxidase catalyzes the reduction of molecular oxygen to superoxide and is essential for microbial defense. Electron transport through the oxidase flavocytochrome is activated by the Rac effector p67(phox). Previous studies suggest that Vav1 regulates NADPH oxidase activity elicited by the chemoattractant formyl-Met-Leu-Phe (fMLP). We show that Vav1 associates with p67(phox) and Rac2, but not Rac1, in fMLP-stimulated human neutrophils, correlating with superoxide production. The interaction of p67(phox) with Vav1 is direct and activates nucleotide exchange on Rac, which enhances the interaction between p67(phox) and Vav1. This provides new molecular insights into regulation of the neutrophil NADPH oxidase, suggesting that chemoattractant-stimulated superoxide production can be amplified by a positive feedback loop in which p67(phox) targets Vav1-mediated Rac activation.
Figures




References
-
- Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. - PubMed
-
- Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. - PubMed
-
- Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. - PubMed
-
- Billadeau, D. D., S. M. Mackie, R. A. Schoon, and P. J. Leibson. 2000. Specific subdomains of Vav differentially affect T cell and NK cell activation. J. Immunol. 164:3971-3981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
