VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail
- PMID: 17060462
- PMCID: PMC1800649
- DOI: 10.1128/MCB.00892-06
VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail
Abstract
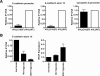
The product of the von Hippel-Lindau gene (VHL) acts as the substrate-recognition component of an E3 ubiquitin ligase complex that ubiquitylates the catalytic alpha subunit of hypoxia-inducible factor (HIF) for oxygen-dependent destruction. Although emerging evidence supports the notion that deregulated accumulation of HIF upon the loss of VHL is crucial for the development of clear-cell renal cell carcinoma (CC-RCC), the molecular events downstream of HIF governing renal oncogenesis remain unclear. Here, we show that the expression of a homophilic adhesion molecule, E-cadherin, a major constituent of epithelial cell junctions whose loss is associated with the progression of epithelial cancers, is significantly down-regulated in primary CC-RCC and CC-RCC cell lines devoid of VHL. Reintroduction of wild-type VHL in CC-RCC (VHL(-/-)) cells markedly reduced the expression of E2 box-dependent E-cadherin-specific transcriptional repressors Snail and SIP1 and concomitantly restored E-cadherin expression. RNA interference-mediated knockdown of HIFalpha in CC-RCC (VHL(-/-)) cells likewise increased E-cadherin expression, while functional hypoxia or expression of VHL mutants incapable of promoting HIFalpha degradation attenuated E-cadherin expression, correlating with the disengagement of RNA polymerase II from the endogenous E-cadherin promoter/gene. These findings reveal a critical HIF-dependent molecular pathway connecting VHL, an established "gatekeeper" of the renal epithelium, with a major epithelial tumor suppressor, E-cadherin.
Figures




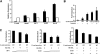

Similar articles
-
Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B.Cancer Res. 2006 Mar 1;66(5):2725-31. doi: 10.1158/0008-5472.CAN-05-3719. Cancer Res. 2006. PMID: 16510593
-
The role of VHL in the regulation of E-cadherin: a new connection in an old pathway.Cell Cycle. 2007 Jan 1;6(1):56-9. doi: 10.4161/cc.6.1.3668. Epub 2007 Jan 5. Cell Cycle. 2007. PMID: 17245122 Review.
-
Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin.Mol Biol Cell. 2009 Feb;20(3):1089-101. doi: 10.1091/mbc.e08-06-0566. Epub 2008 Dec 10. Mol Biol Cell. 2009. PMID: 19073886 Free PMC article.
-
The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma.Oncogene. 2001 Aug 16;20(36):5067-74. doi: 10.1038/sj.onc.1204602. Oncogene. 2001. PMID: 11526493
-
Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer.Am J Nephrol. 2004 Jan-Feb;24(1):1-13. doi: 10.1159/000075346. Epub 2003 Dec 3. Am J Nephrol. 2004. PMID: 14654728 Review.
Cited by
-
Hypoxia-induced epithelial to mesenchymal transition in cancer.Cancer Lett. 2020 Sep 1;487:10-20. doi: 10.1016/j.canlet.2020.05.012. Epub 2020 May 26. Cancer Lett. 2020. PMID: 32470488 Free PMC article. Review.
-
Under-expression of CK2β subunit in ccRCC represents a complementary biomarker of p-STAT3 Ser727 that correlates with patient survival.Oncotarget. 2017 Dec 19;9(5):5736-5751. doi: 10.18632/oncotarget.23422. eCollection 2018 Jan 19. Oncotarget. 2017. PMID: 29464030 Free PMC article.
-
Renal involvement in tuberous sclerosis complex and von Hippel-Lindau disease: shared disease mechanisms?Nat Clin Pract Nephrol. 2009 Mar;5(3):143-56. doi: 10.1038/ncpneph1032. Nat Clin Pract Nephrol. 2009. PMID: 19240728 Review.
-
A dialogue between the hypoxia-inducible factor and the tumor microenvironment.Cancer Microenviron. 2008 Dec;1(1):53-68. doi: 10.1007/s12307-008-0006-3. Epub 2008 Mar 19. Cancer Microenviron. 2008. PMID: 19308685 Free PMC article.
-
Fascin-1 Promotes Cell Metastasis through Epithelial-Mesenchymal Transition in Canine Mammary Tumor Cell Lines.Vet Sci. 2024 May 25;11(6):238. doi: 10.3390/vetsci11060238. Vet Sci. 2024. PMID: 38921985 Free PMC article.
References
-
- Affymetrix. 2003. GeneChip expression analysis technical manual. Affymetrix, Santa Clara, CA.
-
- Ananth, S., B. Knebelmann, W. Gruning, M. Dhanabal, G. Walz, I. Stillman, and V. Sukhatme. 1999. Transforming growth factor beta1 is a target for the von Hippel-Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res. 59:2210-2216. - PubMed
-
- Baba, M., S. Hirai, S. Kawakami, T. Kishida, N. Sakai, S. Kaneko, M. Yao, T. Shuin, Y. Kubota, M. Hosaka, and S. Ohno. 2001. Tumor suppressor protein VHL is induced at high cell density and mediates contact inhibition of cell growth. Oncogene 20:2727-2736. - PubMed
-
- Baba, M., S. Hirai, H. Yamada-Okabe, K. Hamada, H. Tabuchi, K. Kobayashi, K. Kondo, M. Yoshida, A. Yamashita, T. Kishida, N. Nakaigawa, Y. Nagashima, Y. Kubota, M. Yao, and S. Ohno. 2003. Loss of von Hippel-Lindau protein causes cell density dependent deregulation of CyclinD1 expression through hypoxia-inducible factor. Oncogene 22:2728-2738. - PubMed
-
- Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2:84-89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
