Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets
- PMID: 17060476
- PMCID: PMC2118136
- DOI: 10.1084/jem.20061302
Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets
Abstract
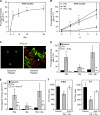
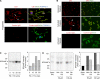
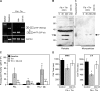
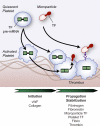
Tissue factor (TF) is an essential cofactor for the activation of blood coagulation in vivo. We now report that quiescent human platelets express TF pre-mRNA and, in response to activation, splice this intronic-rich message into mature mRNA. Splicing of TF pre-mRNA is associated with increased TF protein expression, procoagulant activity, and accelerated formation of clots. Pre-mRNA splicing is controlled by Cdc2-like kinase (Clk)1, and interruption of Clk1 signaling prevents TF from accumulating in activated platelets. Elevated intravascular TF has been reported in a variety of prothrombotic diseases, but there is debate as to whether anucleate platelets-the key cellular effector of thrombosis-express TF. Our studies demonstrate that human platelets use Clk1-dependent splicing pathways to generate TF protein in response to cellular activation. We propose that platelet-derived TF contributes to the propagation and stabilization of a thrombus.
Figures

References
-
- Jurk, K., and B.E. Kehrel. 2005. Platelets: physiology and biochemistry. Semin. Thromb. Hemost. 31:381–392. - PubMed
-
- Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. - PubMed
-
- Roberts, H.R., M. Hoffman, and D.M. Monroe. 2006. A cell-based model of thrombin generation. Semin. Thromb. Hemost. 32(Suppl 1):32–38. - PubMed
-
- Lentz, B.R. 2003. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 42:423–438. - PubMed
-
- Morrissey, J.H., P.F. Neuenschwander, Q. Huang, C.D. McCallum, B. Su, and A.E. Johnson. 1997. Factor VIIa-tissue factor: functional importance of protein-membrane interactions. Thromb. Haemost. 78:112–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

