Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis
- PMID: 17060494
- PMCID: PMC2064562
- DOI: 10.1083/jcb.200606016
Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis
Abstract
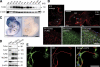
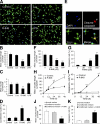
Toll receptors in Drosophila melanogaster function in morphogenesis and host defense. Mammalian orthologues of Toll, the Toll-like receptors (TLRs), have been studied extensively for their essential functions in controlling innate and adaptive immune responses. We report that TLR8 is dynamically expressed during mouse brain development and localizes to neurons and axons. Agonist stimulation of TLR8 in cultured cortical neurons causes inhibition of neurite outgrowth and induces apoptosis in a dissociable manner. Our evidence indicates that such TLR8-mediated neuronal responses do not involve the canonical TLR-NF-kappaB signaling pathway. These findings reveal novel functions for TLR8 in the mammalian nervous system that are distinct from the classical role of TLRs in immunity.
Figures



References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. - PubMed
-
- Belvin, M.P., and K.V. Anderson. 1996. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 12:393–416. - PubMed
-
- Boulanger, L.M., and C.J. Shatz. 2004. Immune signalling in neural development, synaptic plasticity and disease. Nat. Rev. Neurosci. 5:521–531. - PubMed
-
- Coulpier, M., J. Anders, and C.F. Ibanez. 2002. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J. Biol. Chem. 277:1991–1999. - PubMed
-
- Du, X., A. Poltorak, Y. Wei, and B. Beutler. 2000. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur. Cytokine Netw. 11:362–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

